��Ŀ����
�������ϣ��١������ߺš� �ķ������ɾ��ķ���ϩ�Ͳ�����ά����϶��ɣ��ڹ⻯ѧ�����ǵ��������ǵ������������½�����Ҫԭ�۹����ж��Ľ������ܾݻ�����������������ʾ����װ��ס����ȩ�����߳ɳ��ꡣ���ڹ㶫����ۺ�����������ֹ�������ijೱ���ݴ��жϣ�����˵����ȷ����
(1)�������ߺš� �ķ������Ǹ��ϲ��ϣ��ķ���ϩ���ڲ���������
(2)�⻯ѧ��Ⱦ��Ҫ���ɵ��������̼�⻯������ġ�
(3) ����ˮ���¶ȼ�Ӧ������Dz�ɨ�ɾ�
(4)Ϊ���ͼ�ȩ��������װ��ס��Ӧ�����Ŵ���������һ��ˮ
(5)�ೱ��ָ��һ�������º�����ijЩ�����ʱ���ڴ�����ֳ��ۼ���ʹ��ˮ���ɫ����ɫ������
(1)�������ߺš� �ķ������Ǹ��ϲ��ϣ��ķ���ϩ���ڲ���������
(2)�⻯ѧ��Ⱦ��Ҫ���ɵ��������̼�⻯������ġ�
(3) ����ˮ���¶ȼ�Ӧ������Dz�ɨ�ɾ�
(4)Ϊ���ͼ�ȩ��������װ��ס��Ӧ�����Ŵ���������һ��ˮ
(5)�ೱ��ָ��һ�������º�����ijЩ�����ʱ���ڴ�����ֳ��ۼ���ʹ��ˮ���ɫ����ɫ������
| A��(1)(2)(3) | B��(1)(2)(5) | C��(2)(3)(5) | D��(2)(4)(5) |
C
�������������ֻ����̼����Ԫ�أ����ķ���ϩ���ڲ��������Ǵ���ģ���(1)���⻯ѧ��Ⱦ��Ҫ���ɵ��������̼�⻯������ģ���(2)��ȷ������ˮ���¶ȼ�Ӧ�����������������ˮ��������ɨ�ɾ�����(3) ��ȷ��Ϊ���ͼ�ȩ������Ӧ�ô��Ŵ�������Ӧ���ǹر��Ŵ�����(4)���ೱ��ָ��һ�������º�����ijЩ�����ʱ���ڴ�����ֳ��ۼ���ʹ��ˮ���ɫ����ɫ������(5)��ȷ������(2)(3)(5)��˵������ȷ�ģ���ѡ��C��
���������������µĿƼ��ͻ�����Ⱦ��ʵ����ѧ���Ļ�ѧ֪ʶ���п��飬�������ߣ����ͣ���һ�����ۺ��ԣ��Ѷ����С�

��ϰ��ϵ�д�
 �߽�������ϵ�д�
�߽�������ϵ�д�
�����Ŀ
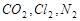 ,����
,����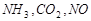 ����
����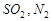 ,�۳�
,�۳�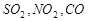 ���̳�
���̳�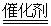 4CO2��N2
4CO2��N2 CO(g)��H2(g)��C(s)��H2(g)��CO(g)��ȫȼ�յ��Ȼ�ѧ����ʽΪ��
CO(g)��H2(g)��C(s)��H2(g)��CO(g)��ȫȼ�յ��Ȼ�ѧ����ʽΪ��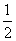 O2(g)===H2O(g) ��H����242.0 kJ/mol ��
O2(g)===H2O(g) ��H����242.0 kJ/mol ��