��Ŀ����
����ʮ�ߴ�ָ��������ǿ��Դ��Դ��Լ����̬������������ǿ�ɳ�����չ��������ֽ�Լ��Դ�ͱ��������Ļ������ߣ���չ������ҵ����
(1)�����й���������������Ҫ�����________��
(2)��úת��Ϊˮú������Ҫ��ѧ��ӦΪ
C(s)��H2O(g) CO(g)��H2(g)��C(s)��H2(g)��CO(g)��ȫȼ�յ��Ȼ�ѧ����ʽΪ��
CO(g)��H2(g)��C(s)��H2(g)��CO(g)��ȫȼ�յ��Ȼ�ѧ����ʽΪ��
C(s)��O2(g)===CO2(g) ��H����393.5 kJ/mol�� ��
H2(g)��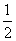 O2(g)===H2O(g) ��H����242.0 kJ/mol ��
O2(g)===H2O(g) ��H����242.0 kJ/mol ��
CO(g)��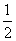 O2(g)===CO2(g) ��H����283.0 kJ/mol�� ��
O2(g)===CO2(g) ��H����283.0 kJ/mol�� ��
��ش�
�ٸ����������ݣ�д��C(s)��H2O(g)��Ӧ���Ȼ�ѧ����ʽ��_____________________________________.
��ˮú������������������ȼ�ϣ�Ҳ����Ҫ���л�����ԭ�ϣ�CO��H2��һ�������¿��Ժϳɣ�a.�״���b.��ȩ��c.���d.���ᣮ�Է�����CO��H2��1��1������Ȼ�Ϸ�Ӧ���ϳ�����________(�����)����ʱ���������㡰��ɫ��ѧ����Ҫ����ȫ����ԭ���е�ԭ�ӣ�ʵ�����ŷţ�
(1)�����й���������������Ҫ�����________��
| A����úҺ�������������ȼ�ϵ�ȼ��Ч�� |
B����װ����β����ת��װ�ã�ʹ֮��Ӧ��4CO��2NO2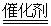 4CO2��N2 4CO2��N2 |
| C�������ƹ��Ҵ����͵�ͬʱ���о�����̫������������ȼ�յ������ |
| D����ˮ�����硢�������硢���ܷ���ͷ���������Ҫ������չ�������� |
C(s)��H2O(g)
 CO(g)��H2(g)��C(s)��H2(g)��CO(g)��ȫȼ�յ��Ȼ�ѧ����ʽΪ��
CO(g)��H2(g)��C(s)��H2(g)��CO(g)��ȫȼ�յ��Ȼ�ѧ����ʽΪ��C(s)��O2(g)===CO2(g) ��H����393.5 kJ/mol�� ��
H2(g)��
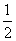 O2(g)===H2O(g) ��H����242.0 kJ/mol ��
O2(g)===H2O(g) ��H����242.0 kJ/mol ��CO(g)��
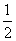 O2(g)===CO2(g) ��H����283.0 kJ/mol�� ��
O2(g)===CO2(g) ��H����283.0 kJ/mol�� ����ش�
�ٸ����������ݣ�д��C(s)��H2O(g)��Ӧ���Ȼ�ѧ����ʽ��_____________________________________.
��ˮú������������������ȼ�ϣ�Ҳ����Ҫ���л�����ԭ�ϣ�CO��H2��һ�������¿��Ժϳɣ�a.�״���b.��ȩ��c.���d.���ᣮ�Է�����CO��H2��1��1������Ȼ�Ϸ�Ӧ���ϳ�����________(�����)����ʱ���������㡰��ɫ��ѧ����Ҫ����ȫ����ԭ���е�ԭ�ӣ�ʵ�����ŷţ�
(1)D (2)��C(s)��H2O(g)===CO(g)��H2(g) ��H����131.5 kJ/mol ��b��d
���������(1)A�н�úҺ��������������Ч���úȼ�ղ��������������ʣ�B�ж�����β�����д��������ϻ���Ҫ��C���ƹ��Ҵ����ͣ������ڼ�����Ⱦ����ŷ�����D�л����������Ҫȼ����ú����ȼ�ղ�����SO2�Ǵ�����Ⱦ�CO2���������壬�����ڻ�����������ѡD��
(2)úת��Ϊˮú���ķ�Ӧ���ɢ٣��ڣ��۵õ����ɵ�C(s)��H2O(g)===CO(g)��H2(g)����H����131.5 kJ/mol��CO��H2��1��1��Ӧ�ϳ�����ʱ���ɴﵽ���ŷţ����ϡ���ɫ��ѧ����Ҫ����ϳɵ����ʵ����ʽӦ����CH2O����b��d���ϣ���ѡbd��
��������ѧ��ɳ�����չ�������������ܲ��ǽ�ѧ���ص㣬�������������ǵ�����������ϢϢ��أ���˳�Ϊ����߿��ıؿ����ȵ㡣����������Ѷȶ��Ի����⡢�е���Ϊ����������ҪΪѡ���⡢�����������ʽ�dz��ѻ�ѧ֪ʶ��ʵ����������������������Ƽ�ǰ�ص�������������ͻ����ѧ��ɳ�����չ������������������ϵ���ۺϿ���ѧ���������⡢��������������

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
