��Ŀ����
��200mL 6mol?L-1�����м���һ�����Ĵ���CaCO3����������������ʱ��ı仯������ͼ��ʾ������������ڱ� ״���²ⶨ����
��ش��������⣺
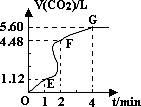
��1����OE�εķ�Ӧ����Ϊ��1��EF�εķ�Ӧ����Ϊ��2��FG�εķ�Ӧ����Ϊ��3�����1����2����3�Ӵ�С��˳��Ϊ______��
��2��Ϊ�˼���������Ӧ�����ʣ��������Һ�м����������ʣ�����Ϊ���е���______������ĸ����
A������ˮ B���Ȼ��ع��� C���Ȼ�����Һ D��Ũ����
��3������CaCO3������Ϊ______��
��4������Ӧ��������Һ����ı仯���Բ��ƣ���EF���������ʾ�Ļ�ѧ��Ӧ���ʦԣ�HCl��=______��
��ش��������⣺

��1����OE�εķ�Ӧ����Ϊ��1��EF�εķ�Ӧ����Ϊ��2��FG�εķ�Ӧ����Ϊ��3�����1����2����3�Ӵ�С��˳��Ϊ______��
��2��Ϊ�˼���������Ӧ�����ʣ��������Һ�м����������ʣ�����Ϊ���е���______������ĸ����
A������ˮ B���Ȼ��ع��� C���Ȼ�����Һ D��Ũ����
��3������CaCO3������Ϊ______��
��4������Ӧ��������Һ����ı仯���Բ��ƣ���EF���������ʾ�Ļ�ѧ��Ӧ���ʦԣ�HCl��=______��
��1����ͼ���֪����λʱ�������ɵ�����EF�Σ�OE�Σ�FG�Σ���λʱ����������������Խ��Ӧ����Խ����
v2��v1��v3���ʴ�Ϊ��v2��v1��v3��
��2��A����������ˮ����Һ��Ũ�ȼ�С����Ӧ���ʼ�С����A��ȷ��
B���Ȼ��ع������Һ��Ũ����Ӱ�죬��KCl���μӷ�Ӧ���������ʲ��䣬��B����
C�������Ȼ�����Һ����С�����Ũ�ȣ���Ӧ���ʼ�С����C��ȷ��
D������Ũ���ᣬ�����Ũ������Ӧ��������D����
�ʴ�Ϊ��AC��
��3������Ӧ���е�4minʱ�����������������䣬˵��̼�����ȫ��Ӧ��
����CaCO3+2HCl�TCaCl2+CO2��+H2O��
100g 22.4L
x 5.60L
����x=
=25g��
�ʴ�Ϊ��25g��
��4��EF�����ɶ�����̼�����Ϊ4.48L-1.12L=3.36L��
CaCO3+2HCl�TCaCl2+CO2��+H2O
2mol 22.4L
n 3.36L
��n=
=0.3mol��
���������Ũ��Ϊc��HCl��=
=1.5mol/L��
����EF���������ʾ�Ļ�ѧ��Ӧ����v��HCl��=
=1.5mol?L-1?min-1��
�ʴ�Ϊ��1.5mol?L-1?min-1��
v2��v1��v3���ʴ�Ϊ��v2��v1��v3��
��2��A����������ˮ����Һ��Ũ�ȼ�С����Ӧ���ʼ�С����A��ȷ��
B���Ȼ��ع������Һ��Ũ����Ӱ�죬��KCl���μӷ�Ӧ���������ʲ��䣬��B����
C�������Ȼ�����Һ����С�����Ũ�ȣ���Ӧ���ʼ�С����C��ȷ��
D������Ũ���ᣬ�����Ũ������Ӧ��������D����
�ʴ�Ϊ��AC��
��3������Ӧ���е�4minʱ�����������������䣬˵��̼�����ȫ��Ӧ��
����CaCO3+2HCl�TCaCl2+CO2��+H2O��
100g 22.4L
x 5.60L
����x=
| 100g��5.60L |
| 22.4L |
�ʴ�Ϊ��25g��
��4��EF�����ɶ�����̼�����Ϊ4.48L-1.12L=3.36L��
CaCO3+2HCl�TCaCl2+CO2��+H2O
2mol 22.4L
n 3.36L
��n=
| 2mol��3.36L |
| 22.4L |
���������Ũ��Ϊc��HCl��=
| 0.3mol |
| 0.2L |
����EF���������ʾ�Ļ�ѧ��Ӧ����v��HCl��=
| 1.5mol/L |
| 1min |
�ʴ�Ϊ��1.5mol?L-1?min-1��

��ϰ��ϵ�д�
�����Ŀ
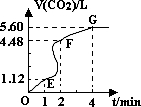
��5�֣���200mL 6mol��L-1�����м���һ�����Ĵ���CaCO3����������������ʱ��ı仯������ͼ��ʾ������������ڱ�״���²ⶨ����
��ش��������⣺
��1����OE�εķ�Ӧ����Ϊ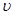 1��EF�εķ�Ӧ����Ϊ
1��EF�εķ�Ӧ����Ϊ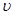 2��FG�εķ�Ӧ����Ϊ
2��FG�εķ�Ӧ����Ϊ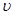 3����
3����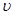 1��
1��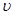 2��
2��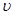 3�Ӵ�С��˳��Ϊ ��
3�Ӵ�С��˳��Ϊ ��
��2��Ϊ�˼���������Ӧ�����ʣ��������Һ�м����������ʣ�����Ϊ���е��� ������ĸ����
| A������ˮ | B���Ȼ��ع��� |
| C���Ȼ�����Һ | D��Ũ���� |
��4������Ӧ��������Һ����ı仯���Բ��ƣ���EF���������ʾ�Ļ�ѧ��Ӧ����
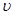 ��HCl��= ��
��HCl��= �� ( 8�� )��200mL 6mol��L-1�����м���һ�����Ĵ���CaCO3����������������ʱ��ı仯������ͼ��ʾ������������ڱ�״���²ⶨ������ش��������⣺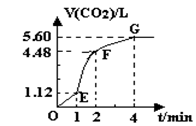
��1����OE�εķ�Ӧ����Ϊv1��EF�εķ�Ӧ����Ϊv2��FG�εķ�Ӧ����Ϊv3����v1��v2��v3�Ӵ�С��˳��Ϊ
��2��Ϊ�˼���������Ӧ�����ʣ��������Һ�м����������ʣ�����Ϊ���е��� ������ĸ����
| A������ˮ | B���Ȼ��ع��� | C���Ȼ�����Һ | D��Ũ���� |
��4������Ӧ��������Һ����ı仯���Բ��ƣ���EF���������ʾ�Ļ�ѧ��Ӧ����V��HCl��== ��
 A������ˮ B���Ȼ��ع��� C���Ȼ�����Һ D��Ũ����
A������ˮ B���Ȼ��ع��� C���Ȼ�����Һ D��Ũ����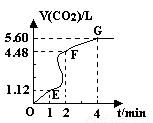 A������ˮ B���Ȼ��ع��� C���Ȼ�����Һ D��Ũ����
A������ˮ B���Ȼ��ع��� C���Ȼ�����Һ D��Ũ����