��Ŀ����
�������Ƿ��Ϳɵ����ᣬ��ţ����Ҳ����ȡ���ᣬ��������Ϊ��ɫ��Һ�壬������ˮ��Ϊ���о�����ķ�����ɺͽṹ����������ʵ�飺
��1����ȡ����0��90 g����ij��״����ʹ����ȫ��������֪��ͬ״����ͬ�������Ϊ0��02 g�����������Է�������Ϊ_________________��
��2��������������������O2��ȼ��ֻ����CO2��H2O��g������ȫ������ʯ������ʱ����ʯ������1��86 g������������ͨ������ʯ��ˮ����ʯ��ˮ�����3��00 g ��ɫ������������ķ���ʽΪ_________________��
��3����������ܷ�������������Ӧ����������ﲻ�ܷ���������Ӧ�������Ƿ���ֻ�������ᣬ��д����������������Ļ�ѧ����ʽ��________________________________________��
��4��д�������ڴ��������·�����Ӧ�����ɷ���ʽΪC6H8O4�Ļ�״���Ľṹ��ʽ��
__________________________________��
��1��90
��2��C3H6O3
��3��CH2OH��CHOH��4CHO��2CH3CH��OH��COOH
��4��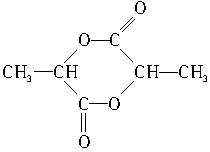
����:
��1���������Է��������ɰ����ӵ����ɿ��Ƴ���
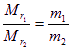 ������
������![]() ��2=90��
��2=90��
��2��0��01 mol������ȫȼ������CO2 0��03 mol������H2O��
![]() =0��03 mol��
=0��03 mol��
����һ�������������3��̼ԭ�Ӻ�6��Hԭ�ӣ���������Է�������Ϊ90���ʺ���ԭ����Ϊ![]() =3����������ķ���ʽΪC3H6O3��
=3����������ķ���ʽΪC3H6O3��
��3��������ķ���ʽ�������Ϣ�ƶϣ�����Ľṹ��ʽΪ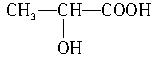 ���������Ƿ�����������Ļ�ѧ����ʽΪ��CH2OH��CHOH��4CHO��2CH3CH��OH��COOH
���������Ƿ�����������Ļ�ѧ����ʽΪ��CH2OH��CHOH��4CHO��2CH3CH��OH��COOH
��4��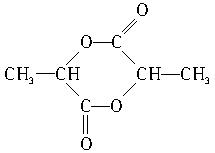

 ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д�