��Ŀ����
���и���������ָ����Һ��һ���ܴ���������ǣ�������
����ɫ��Һ�У�K+��Na+��MnO4-��SO42-
��pH=11����Һ�У�CO32-��Na+��NO3-��[Al��OH��4]-
�ۼ���Al�ܷų�H2����Һ�У�Cl-��SO42-��NO3-��Mg2+
������ˮ�������c��OH-��=10-13mol?L-1����Һ�У�Na+��Ba2+��Cl-��I-
������Һ���ܴ������棬����NaOH����ȼ�������ų����г������ɵ���Һ��Ca2+��HCO3-��NH4+��Al3+
����ʹ��̪���ɫ����Һ��Na+��C1-��S2-��SO32-��
����ɫ��Һ�У�K+��Na+��MnO4-��SO42-
��pH=11����Һ�У�CO32-��Na+��NO3-��[Al��OH��4]-
�ۼ���Al�ܷų�H2����Һ�У�Cl-��SO42-��NO3-��Mg2+
������ˮ�������c��OH-��=10-13mol?L-1����Һ�У�Na+��Ba2+��Cl-��I-
������Һ���ܴ������棬����NaOH����ȼ�������ų����г������ɵ���Һ��Ca2+��HCO3-��NH4+��Al3+
����ʹ��̪���ɫ����Һ��Na+��C1-��S2-��SO32-��
����������ɫ��Һ�в���������ɫ�����ӣ�
��pH=11����Һ�ʼ��ԣ�
�ۼ���Al�ܷų�H2����Һ���ܳ����ԣ�Ҳ���ܳʼ��ԣ�
����ˮ�������c��OH-��=10-13mol?L-1����Һ��ˮ�ĵ����ܵ����ƣ���Һ���ܳ����ԣ�Ҳ���ܳʼ��ԣ�
��HCO3-��Al3+��������ˮ�ⷴӦ�����ܴ������棻
����ʹ��̪���ɫ����Һ�ʼ��ԣ�
��pH=11����Һ�ʼ��ԣ�
�ۼ���Al�ܷų�H2����Һ���ܳ����ԣ�Ҳ���ܳʼ��ԣ�
����ˮ�������c��OH-��=10-13mol?L-1����Һ��ˮ�ĵ����ܵ����ƣ���Һ���ܳ����ԣ�Ҳ���ܳʼ��ԣ�
��HCO3-��Al3+��������ˮ�ⷴӦ�����ܴ������棻
����ʹ��̪���ɫ����Һ�ʼ��ԣ�
����⣺��MnO4-����ɫ����������ĿҪ�ʢٴ���
��pH=11����Һ�ʼ��ԣ�����֮�䲻�����κη�Ӧ���ɴ������棬�ʢ���ȷ��
�ۼ���Al�ܷų�H2����Һ���ܳ����ԣ�Ҳ���ܳʼ��ԣ�������Һ�в��������������ʢ۴���
����ˮ�������c��OH-��=10-13mol?L-1����Һ��ˮ�ĵ����ܵ����ƣ���Һ���ܳ����ԣ�Ҳ���ܳʼ��ԣ��������Ի��Ǽ��ԣ�����֮�䶼�������κη�Ӧ���ɴ������棬�ʢ���ȷ��
��HCO3-��Al3+��������ˮ�ⷴӦ�����ܴ������棬�ʢݴ���
����ʹ��̪���ɫ����Һ�ʼ��ԣ��ڼ�������������֮�䲻�����κη�Ӧ���ɴ������ڣ��ʢ���ȷ��
��ѡC��
��pH=11����Һ�ʼ��ԣ�����֮�䲻�����κη�Ӧ���ɴ������棬�ʢ���ȷ��
�ۼ���Al�ܷų�H2����Һ���ܳ����ԣ�Ҳ���ܳʼ��ԣ�������Һ�в��������������ʢ۴���
����ˮ�������c��OH-��=10-13mol?L-1����Һ��ˮ�ĵ����ܵ����ƣ���Һ���ܳ����ԣ�Ҳ���ܳʼ��ԣ��������Ի��Ǽ��ԣ�����֮�䶼�������κη�Ӧ���ɴ������棬�ʢ���ȷ��
��HCO3-��Al3+��������ˮ�ⷴӦ�����ܴ������棬�ʢݴ���
����ʹ��̪���ɫ����Һ�ʼ��ԣ��ڼ�������������֮�䲻�����κη�Ӧ���ɴ������ڣ��ʢ���ȷ��
��ѡC��
���������⿼�����ӹ������⣬��Ŀ�Ѷ��еȣ�����ע�����Ҫ��Ϊ������Ĺؼ��������״���Ϊ�ۣ�ע�������ǿ�����ԣ�

��ϰ��ϵ�д�
�����Ŀ
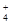 ��Na+��HSO
��Na+��HSO ��PO
��PO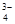 ��MnO
��MnO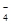
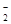 ��NO
��NO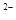 ��SO
��SO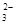
 ��CO
��CO ��Cl
��Cl =0.1 mol/L����Һ�У�Na+��K+��SO
=0.1 mol/L����Һ�У�Na+��K+��SO