��Ŀ����
��ÿ��1�֣���6�֣�������ij��ѧ��ȤС���ͬѧ��ͬ����Ԫ�����ʵݱ����ʵ��ʱ����Ƶ�һ��ʵ�鷽��������ʵ�鲽�����£�

�����������ʵ�鱨�棺
��1��ʵ��Ŀ�ģ� ��
��2��ʵ����Ʒ��
�Լ��������ơ�þ����������ϡ���ᡢ������ˮ������Na2S��Һ��AlCl3��Һ��NaOH��Һ�ȡ�
������ ���ƾ��ơ��������ԹܼС����ӡ�С��������Ƭ��ɰֽ�ȡ�
��3��ʵ�����ݣ�������б����е�δ��ɵ�ʵ������ͻ�ѧ����ʽ����
��4��ʵ����ۣ� ��
��5������ԭ�ӽṹ��֪ʶ�����������ۣ� ��

�����������ʵ�鱨�棺
��1��ʵ��Ŀ�ģ� ��
��2��ʵ����Ʒ��
�Լ��������ơ�þ����������ϡ���ᡢ������ˮ������Na2S��Һ��AlCl3��Һ��NaOH��Һ�ȡ�
������ ���ƾ��ơ��������ԹܼС����ӡ�С��������Ƭ��ɰֽ�ȡ�
��3��ʵ�����ݣ�������б����е�δ��ɵ�ʵ������ͻ�ѧ����ʽ����
| ʵ����� | ʵ������ | ��ѧ����ʽ |
| �� | �������������Һ���dz��ɫ | |
| �� | ����dz��ɫ���� | Na2S��Cl2��2NaCl��S�� |
| �� | | 2Na��2H2O��2NaOH��H2�� |
| �� | ���ҷ�Ӧ��Ѹ�ٲ�����ɫ���� | Mg��2HCl��MgCl2��H2�� |
| �� | ��Ӧ��ʮ�־��ң�������ɫ���� | 2Al��6HCl��2AlCl3��3H2�� |
| �� | ��ʼʱ���ɰ�ɫ��״�������̶�������ʧ | AlCl3��3NaOH��Al(OH)3����3NaCl Al(OH)3��NaOH��NaAlO2��2H2O |
��5������ԭ�ӽṹ��֪ʶ�����������ۣ� ��
��ÿ��2�֣���6�֣�
��1��ʵ��Ŀ�ģ�̽��ͬ����Ԫ�����ʵĵݱ����
��2��ʵ����Ʒ���������Թ�
��3��ʵ�����ݣ�
ʵ��������ˮ���ϣ��۳�С��������ų������������ƶ�����֮��ʧ����Һ��ɺ�ɫ
��ѧ����ʽ��Mg+2H2O Mg(OH) 2+2H2��
Mg(OH) 2+2H2��
��4��ʵ����ۣ�ͬ����Ԫ����ԭ�������ĵ�����ʧ���������������õ�������������ǿ��
��5������ԭ�ӽṹ��֪ʶ�����������ۣ���ͬ����Ԫ�ص�ԭ�ӵ��Ӳ�����ȣ��˵������С��ԭ�Ӻ˶Ժ�����ӵ�������������ǿ�����������������õ�������������ǿ��
��1��ʵ��Ŀ�ģ�̽��ͬ����Ԫ�����ʵĵݱ����
��2��ʵ����Ʒ���������Թ�
��3��ʵ�����ݣ�
ʵ��������ˮ���ϣ��۳�С��������ų������������ƶ�����֮��ʧ����Һ��ɺ�ɫ
��ѧ����ʽ��Mg+2H2O
 Mg(OH) 2+2H2��
Mg(OH) 2+2H2����4��ʵ����ۣ�ͬ����Ԫ����ԭ�������ĵ�����ʧ���������������õ�������������ǿ��
��5������ԭ�ӽṹ��֪ʶ�����������ۣ���ͬ����Ԫ�ص�ԭ�ӵ��Ӳ�����ȣ��˵������С��ԭ�Ӻ˶Ժ�����ӵ�������������ǿ�����������������õ�������������ǿ��
��1������ʵ�鲽���ԭ����֪��ʵ��Ŀ����̽��ͬ����Ԫ�����ʵĵݱ���ɡ�
��2������ʵ�鲽���֪��ʵ����Ҫ�Թܡ�
��3����þ�ǻ��õĽ������ڼ��ȵ��������ܺͷ�ˮ��Ӧ����������������þ������ʽΪMg+2H2O Mg(OH) 2+2H2����
Mg(OH) 2+2H2����
�����ǻ��õĽ���������ˮ��Ӧ�����������������ƣ�����ʵ�������Ǹ���ˮ���ϣ��۳�С��������ų������������ƶ�����֮��ʧ����Һ��ɺ�ɫ��
��4������ʵ�������֪������Ӧ����ͬ����Ԫ����ԭ�������ĵ�����ʧ���������������õ�������������ǿ��
��5������ͬ����Ԫ�ص�ԭ�ӵ��Ӳ�����ȣ��˵������С��ԭ�Ӻ˶Ժ�����ӵ�������������ǿ��ʧȥ���ӵ������������õ�������������ǿ�����Խ������������ǽ���������ǿ��
��2������ʵ�鲽���֪��ʵ����Ҫ�Թܡ�
��3����þ�ǻ��õĽ������ڼ��ȵ��������ܺͷ�ˮ��Ӧ����������������þ������ʽΪMg+2H2O
 Mg(OH) 2+2H2����
Mg(OH) 2+2H2���������ǻ��õĽ���������ˮ��Ӧ�����������������ƣ�����ʵ�������Ǹ���ˮ���ϣ��۳�С��������ų������������ƶ�����֮��ʧ����Һ��ɺ�ɫ��
��4������ʵ�������֪������Ӧ����ͬ����Ԫ����ԭ�������ĵ�����ʧ���������������õ�������������ǿ��
��5������ͬ����Ԫ�ص�ԭ�ӵ��Ӳ�����ȣ��˵������С��ԭ�Ӻ˶Ժ�����ӵ�������������ǿ��ʧȥ���ӵ������������õ�������������ǿ�����Խ������������ǽ���������ǿ��

��ϰ��ϵ�д�
 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
�����Ŀ

 ��Һ�С�
��Һ�С� ��Ũ�����еĶۻ�
��Ũ�����еĶۻ� ��Һ����ȡ����ʳ��
��Һ����ȡ����ʳ��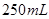 ����ƿ�У���ˮϡ�����̶��ߣ�ҡ�ȵô���ʳ����Һ
����ƿ�У���ˮϡ�����̶��ߣ�ҡ�ȵô���ʳ����Һ
