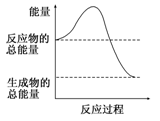
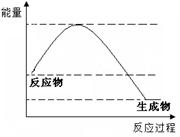
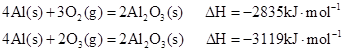��Ŀ����
�����ȼ���(��ͼ)��100 mL 0.50 mol��L��1��CH3COOH��Һ��100 mL 0.55 mol��L��1��NaOH��Һ��ϣ��¶ȴ�298.0 K������300.7 K����֪���ȼƵ����ݳ���(���ȼƸ�����ÿ����1 K����Ҫ����)��150.5 J��K��1����Һ�ܶȾ�Ϊ1 g��mL��1��������Һ�ı�����c��4.184 J��(g��K)��1��
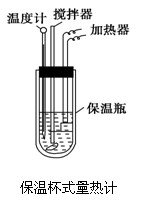
(1).����CH3COOH���к��Ȧ�H������ֵʽ��
(2).������ֵ�����57.3 kJ/mol��ƫ�����ԭ�������
a��ʵ��װ�ñ��¡�����Ч����
b������0.55 mol/L NaOH��Һʱ���ӿ̶��߶���
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
e������Ͳ��ȡNaOH��Һ�����ʱ���Ӷ���

(1).����CH3COOH���к��Ȧ�H������ֵʽ��
(2).������ֵ�����57.3 kJ/mol��ƫ�����ԭ�������
a��ʵ��װ�ñ��¡�����Ч����
b������0.55 mol/L NaOH��Һʱ���ӿ̶��߶���
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
e������Ͳ��ȡNaOH��Һ�����ʱ���Ӷ���
(1) CH3COOH���к��ȣ���H����53.3 kJ��mol��1��
��2��a c d����ѡ����ѡ�������֣�
��2��a c d����ѡ����ѡ�������֣�
��1����Ӧ�зų���������4.184 J��(g��K)��1��200g��2.7K��150.5J��K��1��2.7K��2665.71J�������ڷ�Ӧ������0.05molˮ�����Ը÷�Ӧ���к��ȡ�H����2.66571kJ��0.05mol����53.3 kJ��mol��1��
��2��������ֵ�����57.3 kJ/mol��ƫ���˵����Ӧ����������ʧ������ѡ��acd������ȷ�ģ�����0.55 mol/L NaOH��Һʱ���ӿ̶��߶���������ҺŨ��ƫ�ⶨ���Ӧ����ƫ�͵ģ�����Ͳ��ȡNaOH��Һ�����ʱ���Ӷ�����������������Һ�����ƫ�����Բⶨ���ƫ�ߣ���ѡacd��
��2��������ֵ�����57.3 kJ/mol��ƫ���˵����Ӧ����������ʧ������ѡ��acd������ȷ�ģ�����0.55 mol/L NaOH��Һʱ���ӿ̶��߶���������ҺŨ��ƫ�ⶨ���Ӧ����ƫ�͵ģ�����Ͳ��ȡNaOH��Һ�����ʱ���Ӷ�����������������Һ�����ƫ�����Բⶨ���ƫ�ߣ���ѡacd��

��ϰ��ϵ�д�
 ��������ϵ�д�
��������ϵ�д� ���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
�����Ŀ
 SO3(g)+NO(g) �ﵽƽ�⣬����Ӧ������ʱ��仯����ͼ��ʾ������ȷ�Ľ�����
SO3(g)+NO(g) �ﵽƽ�⣬����Ӧ������ʱ��仯����ͼ��ʾ������ȷ�Ľ�����
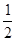 O2 ( g ) �� H2O ( g ) ��H1 =" a" kJ/mol
O2 ( g ) �� H2O ( g ) ��H1 =" a" kJ/mol