��Ŀ����
����Ŀ�����Ĺ̶��Ǽ���������ѧ��һֱ�о��Ŀ��⡣��ش��������⣺
(1)�±��о��˲�ͬ�¶��´����̵���ҵ�̵��IJ���Kֵ��
��Ӧ | �����̵� N2 (g)+O2 (g) | ��ҵ�̵� N2 (g)+3H2 (g) | |||
�¶�/�� | 27 | 2000 | 25 | 400 | 450 |
K | 3.84��10��31 | 0.1 | 5��108 | 0.507 | 0.152 |
���������ݿ�֪�������̵���Ӧ���� ________(����ȡ����ȡ�)��Ӧ��
���������ݿ�֪������ʺϴ��ģģ������̵���ԭ���� ________��
�۴�ƽ���ӽǿ��ǣ���ҵ�̵�Ӧ��ѡ������������ʵ�ʹ�ҵ����ȴѡ��500�����ҵĸ��£�������ԭ�� ________��
(2)��֪��ҵ�̵���Ӧ��N2��g��+ 3H2��g��![]() 2NH3��g����H ����94.4kJ��mol��1������ʱ����ϵ�и�����Ũ����ʱ��仯��������ͼ1��ʾ����ʱ������վ���ƽ��״̬��
2NH3��g����H ����94.4kJ��mol��1������ʱ����ϵ�и�����Ũ����ʱ��仯��������ͼ1��ʾ����ʱ������վ���ƽ��״̬��
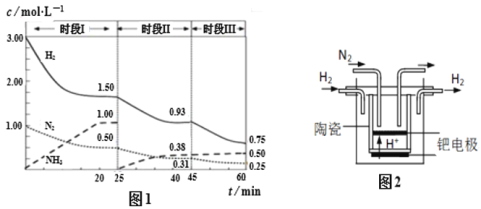
����2L�����з�����Ӧ��ǰ20min�ڣ���(NH3) �� ________��
��25 minʱ��ȡ��ij�ִ�ʩ�� ________��
��ʱ��III�����·�Ӧ��ƽ�ⳣ��Ϊ ________ L2�� mol��2(����3λ��Ч����) ��
(3)20����ĩ����ѧ�Ҳ��ø����ӵ����Ե�SCY�մ�(�ܴ���H+)Ϊ���ʣ�������������������ϵĽ����ٶྦྷ��Ĥ���缫��ʵ�ָ��³�ѹ�µĵ�ⷨ�ϳɰ�������˷�Ӧ���ת���ʣ���ʵ���ͼ��ͼ2��ʾ�������ĵ缫��Ӧʽ�� ________��
(4)���꣬���п�ѧ������ڳ��¡���ѹ�������������ºϳɰ�������˼·����Ӧԭ��Ϊ��2N2(g)+6H2O(l)![]() 4NH3(g)+3O2(g)�����䷴Ӧ����H �� ________����֪��N2(g)+ 3H2(g)
4NH3(g)+3O2(g)�����䷴Ӧ����H �� ________����֪��N2(g)+ 3H2(g)![]() 2NH3(g) ��H1 ����92.4kJ��mol��1 2H2(g) +O2(g)
2NH3(g) ��H1 ����92.4kJ��mol��1 2H2(g) +O2(g)![]() 2H2O(l) ��H2 ����571.6kJ��mol��1
2H2O(l) ��H2 ����571.6kJ��mol��1
���𰸡����� KֵС��������еij̶�С����ת���ʵͣ������ʺϴ��ģ���� �ӷ�Ӧ���ʽǶȿ��ǣ����¸��ã����Ӵ������Ե��ۺ����ؿ���ѡ��500�����Һ��� 0.050����0.05��mol/��L��min�� ��NH3�ӷ�Ӧ��ϵ�з����ȥ 2.37 N2+6e-+6H+=2NH3 +1530.0 kJ��mol��1
��������
��1�����¶�Խ�ߣ�KԽ��˵�������¶ȣ�ƽ�����ƣ�
��Kֵ��С��ת���ʺ�С��
�۴�Ӱ�컯ѧ��Ӧ�����뻯ѧƽ��ĽǶȼ�����Ч�����ʵ�ʲ������⣻
��2���ٸ�����= ![]() �����ǰ20min�ڰ�����ƽ����Ӧ������(NH3)��
�����ǰ20min�ڰ�����ƽ����Ӧ������(NH3)��
�ڸ���25minʱ���������ʵ�����Ϊ0�������������������ʵ���������н�𣬸ı�������Ƿ����������
�۸���ʱ�������´ﵽƽ��ʱ����ֵ�Ũ�ȼ�ƽ�ⳣ������������ƽ��Ũ���ݴη��˻����Է�Ӧ��ƽ��Ũ���ݴη��˻����н��
��3�������������õ������ɰ�����
��4����֪����N2��g��+3H2��g��2NH3��g����H=-92.4kJmol-1��
��2H2��g��+O2��g��2H2O��l����H=-571.6kJmol-1��
�ɸ�˹���ɣ�����2-����3��2N2��g��+6H2O��l��4NH3��g��+3O2��g�����ݴ˷�����
��1�����ɱ������ݿ�֪���¶�Խ�ߣ�KԽ��˵�������¶ȣ�ƽ�����ƣ�������Ӧ����Ϊ���ȷ�Ӧ��
�ʴ�Ϊ�����ȣ�
���ɱ������ݿ�֪��2000��ʱ��K=0.1��Kֵ��С����ת���ʺ�С�����ʺϴ��ģ��������������ʺϴ��ģģ������̵���
�ʴ�Ϊ��KֵС��������еij̶�С(��ת���ʵ�)�����ʺϴ��ģ������
�۽�ϱ�����Կ�������ҵ�̵��ķ�Ӧƽ�ⳣ�������¶ȵ����߶����ͣ�Ϊ���ȷ�Ӧ�����¶����ߣ���ѧ��Ӧ���ʻ���������Ҳ���¶�Ӱ�죬���ʵ����¶��»�������ۺϿ��ǣ���ѡ���¶�Ϊ500�����Һ��ʣ���ԭ��ɽ���Ϊ���ӷ�Ӧ���ʽǶȿ��ǣ����¸��ã����Ӵ������Ե��ۺ����ؿ���ѡ��500�����Һ��ʣ�
�ʴ�Ϊ���ӷ�Ӧ���ʽǶȿ��ǣ����¸��ã����Ӵ������Ե��ۺ����ؿ���ѡ��500�����Һ��ʣ�
��2���ٸ���ͼ���֪��20minʱ���������ʵ���Ũ��Ϊ1.00 mol/L����������ƽ����Ӧ����Ϊ����(NH3) =![]() =
= ![]() = 0.050����0.05��mol/��L��min��
= 0.050����0.05��mol/��L��min��
�ʴ�Ϊ��0.050����0.05��mol/��L��min����
��25 minʱ���������ʵ���Ѹ�ٱ�Ϊ0�����������������ʵ�������,֮���������������ʵ�����С�����������ʵ���������˵��25 minʱ�ı�������ǽ�NH3�ӷ�Ӧ��ϵ�з����ȥ��
�ʴ�Ϊ����NH3�ӷ�Ӧ��ϵ�з����ȥ��
��ʱ�������£��䷴ӦΪ��N2(g)+3H2(g)2NH3(g)��ͼ�����֪ƽ��״̬��c(N2) = 0.25 mol/L��c(NH3) = 0.50 mol/L��c(H2) = 0.75 mol/L����÷�Ӧ�Ļ�ѧƽ�ⳣ��Ϊ��K=![]() =
= ![]() =
= ![]() 2.37
2.37
�ʴ�Ϊ��2.37��
(3)�����е����������õ������ɰ�������缫����ʽΪ��N2+6e-+6H+=2NH3��
�ʴ�Ϊ��N2+6e-+6H+=2NH3��
(4)��֪����N2(g)+ 3H2(g)![]() 2NH3(g) ��H1 ����92.4 kJ��mol��1��
2NH3(g) ��H1 ����92.4 kJ��mol��1��
��2H2(g) +O2(g)![]() 2H2O(l) ��H2 ����571.6 kJ��mol��1��
2H2O(l) ��H2 ����571.6 kJ��mol��1��
�ɸ�˹���ɣ�����2����3��2N2(g)+6H2O(l)![]() 4NH3(g)+3O2(g)�����H=(92.4 kJml1)��2(571.6 kJmol1)��3 = + 1530.0 kJmol1��
4NH3(g)+3O2(g)�����H=(92.4 kJml1)��2(571.6 kJmol1)��3 = + 1530.0 kJmol1��
�ʴ�Ϊ��+ 1530.0 kJmol1��

 ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�