��Ŀ����
��12�֣�20 L���ݵ��ܱ������У�����3 mol SO3(g)��1 mol��������ij�¶���ʹ�䷴Ӧ����Ӧ��4 minʱ��������Ũ��Ϊ0.06 mol/L������Ӧ��8 minʱ����Ӧ����ƽ�⡣
(1)0 min��4 min������O2��ƽ�����ʣ�
v(O2)��____________mol/(L��min)��
(2)���������У������ʵ�Ũ����ʱ���ϵ����ͼ��ʾ������¶��µ�ƽ�ⳣ��K��________________��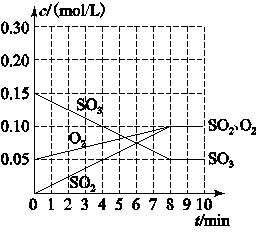
(3)����ʼʱ���±�����Ͷ�ϣ���ͬ�¶��´ﵽƽ��ʱ����������Ũ�ȴ���0.05 mol/L����________����ʱ��ƽ�ⳣ����(2)С��Ƚ�________(����ڡ�����С�ڡ����ڡ�)��
| | A | B | C | D |
| SO3 | 1 mol | 3 mol | 3 mol | 0 mol |
| SO2 | 2 mol | 1.5 mol | 0 mol | 6 mol |
| O2 | 2 mol | 1 mol | 0 mol | 5 mol |
����ϵ��ѹǿ���ٸı�
��������������ܶȲ��ٸı�
�ۻ�������ƽ����Է����������ٸı�
��v��(SO3)��2v��(O2)
��n(SO3)��n(O2)��n(SO2)��2��1��2
(1)0.0025 mol��L��1��min��1
(2)0.4 mol��L��1
(3)BD�����ڡ�(4)�٢ۢ�
����

 ��Ȥ����¹�֪��ϵ�д�
��Ȥ����¹�֪��ϵ�д� Ӣ��СӢ������Ĭдϵ�д�
Ӣ��СӢ������Ĭдϵ�д� �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д���12�֣�20 L���ݵ��ܱ������У�����3 mol SO3(g)��1 mol��������ij�¶���ʹ�䷴Ӧ����Ӧ��4 minʱ��������Ũ��Ϊ0.06mol/L������Ӧ��8 minʱ����Ӧ����ƽ�⡣
(1)0 min��4min������O2��ƽ�����ʣ�
v(O2)��____________mol/(L��min)��
(2)���������У������ʵ�Ũ����ʱ���ϵ����ͼ��ʾ������¶��µ�ƽ�ⳣ��K��________________��
(3)����ʼʱ���±�����Ͷ�ϣ���ͬ�¶��´ﵽƽ��ʱ����������Ũ�ȴ���0.05 mol/L����________����ʱ��ƽ�ⳣ����(2)С��Ƚ�________(����ڡ�����С�ڡ����ڡ�)��
|
| A | B | C | D |
| SO3 | 1 mol | 3 mol | 3 mol | 0 mol |
| SO2 | 2 mol | 1.5 mol | 0 mol | 6 mol |
| O2 | 2 mol | 1 mol | 0 mol | 5 mol |
(4)���ʵ�Ũ�Ȳ��ٸı��־�÷�Ӧ�Ѵ�ƽ�⣬���л�����˵���÷�Ӧ�Ѵ�ƽ�����________(�����)��
����ϵ��ѹǿ���ٸı�
��������������ܶȲ��ٸı�
�ۻ�������ƽ����Է����������ٸı�
��v��(SO3)��2v��(O2)
��n(SO3)��n(O2)��n(SO2)��2��1��2
��12�֣�20 L���ݵ��ܱ������У�����3 mol SO3(g)��1 mol��������ij�¶���ʹ�䷴Ӧ����Ӧ��4 minʱ��������Ũ��Ϊ0.06 mol/L������Ӧ��8 minʱ����Ӧ����ƽ�⡣
(1)0 min��4 min������O2��ƽ�����ʣ�
v(O2)��____________mol/(L��min)��
(2)���������У������ʵ�Ũ����ʱ���ϵ����ͼ��ʾ������¶��µ�ƽ�ⳣ��K��________________��

(3)����ʼʱ���±�����Ͷ�ϣ���ͬ�¶��´ﵽƽ��ʱ����������Ũ�ȴ���0.05 mol/L����________����ʱ��ƽ�ⳣ����(2)С��Ƚ�________(����ڡ�����С�ڡ����ڡ�)��
|
|
A |
B |
C |
D |
|
SO3 |
1 mol |
3 mol |
3 mol |
0 mol |
|
SO2 |
2 mol |
1.5 mol |
0 mol |
6 mol |
|
O2 |
2 mol |
1 mol |
0 mol |
5 mol |
(4)���ʵ�Ũ�Ȳ��ٸı��־�÷�Ӧ�Ѵ�ƽ�⣬���л�����˵���÷�Ӧ�Ѵ�ƽ�����________(�����)��
����ϵ��ѹǿ���ٸı�
��������������ܶȲ��ٸı�
�ۻ�������ƽ����Է����������ٸı�
��v��(SO3)��2v��(O2)
��n(SO3)��n(O2)��n(SO2)��2��1��2
 2NH3(g)�� ��H=-92.4kJ��mol-1��
2NH3(g)�� ��H=-92.4kJ��mol-1�� 
 2NH3(g)��ƽ�ⳣ��K= ________��������С��һλ��KֵԽ������Ӧ�ﵽƽ��ʱ_________�����ţ���
2NH3(g)��ƽ�ⳣ��K= ________��������С��һλ��KֵԽ������Ӧ�ﵽƽ��ʱ_________�����ţ���
 2NH3(g) + O2(g)����H = a kJ��mol-1 ��һ���о�NH3���������¶ȵĹ�ϵ����ѹ�´ﵽƽ��ʱ��ò���ʵ���������±�
2NH3(g) + O2(g)����H = a kJ��mol-1 ��һ���о�NH3���������¶ȵĹ�ϵ����ѹ�´ﵽƽ��ʱ��ò���ʵ���������±� 
 2NH3(g) ��H= ��92 .4kJ��mol-1
2NH3(g) ��H= ��92 .4kJ��mol-1 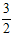 O2(g) ��H=_____________kJ��mol-1
O2(g) ��H=_____________kJ��mol-1  O2��g���TH2O��l����H2=-285.8kJ/mol
O2��g���TH2O��l����H2=-285.8kJ/mol