��Ŀ����
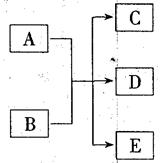
����A��E��ת����ϵ��ͼ��ʾ:
��֪���嵥��D��ʹ�����ǵ�ľ����ȼ,��Ӧ����ʵ�����Ƶ���D�ij��÷���֮һ,��Ӧ����ʵ�����ƻ���ɫ����E����Ҫ��Ӧ,(����������δ�г�)��
����������Ϣ,�ش��������⣺
(1)A�Ļ�ѧʽ
(2) ����Ԫ�ػ��ϼ۵ı仯��������д����Ӧ�ڢۢܢ����ַ����Ĺ�ͬ����
(3)д����Ӧ�ܵĻ�ѧ����ʽ
(4)���÷�Ӧ�ۢܢݵı仯����д��ѧ����ʽ�Ƚ�A��C��D���������ɴ�С��˳��
(5)д����Ӧ�ڵ����ӷ���ʽ ,�������뻹ԭ�������ʵ���֮��

��֪���嵥��D��ʹ�����ǵ�ľ����ȼ,��Ӧ����ʵ�����Ƶ���D�ij��÷���֮һ,��Ӧ����ʵ�����ƻ���ɫ����E����Ҫ��Ӧ,(����������δ�г�)��
����������Ϣ,�ش��������⣺
(1)A�Ļ�ѧʽ
(2) ����Ԫ�ػ��ϼ۵ı仯��������д����Ӧ�ڢۢܢ����ַ����Ĺ�ͬ����
(3)д����Ӧ�ܵĻ�ѧ����ʽ
(4)���÷�Ӧ�ۢܢݵı仯����д��ѧ����ʽ�Ƚ�A��C��D���������ɴ�С��˳��
(5)д����Ӧ�ڵ����ӷ���ʽ ,�������뻹ԭ�������ʵ���֮��
��1��KMnO4
��2����Ԫ�صĻ��ϼ���-1�����ߵ�0��
��3��4HCl+O2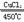 2Cl2+2H2O
2Cl2+2H2O
��4��KMnO4>MnO2>O2
(5) MnO2��4H++2Cl- Mn2++Cl2��+2H2O�� 1:2
Mn2++Cl2��+2H2O�� 1:2
��2����Ԫ�صĻ��ϼ���-1�����ߵ�0��
��3��4HCl+O2
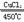 2Cl2+2H2O
2Cl2+2H2O��4��KMnO4>MnO2>O2
(5) MnO2��4H++2Cl-
 Mn2++Cl2��+2H2O�� 1:2
Mn2++Cl2��+2H2O�� 1:2������������������ʵ�����Ʊ�Ϊ�زģ�ע�������Ʊ�������Ϊ������ԭ��Ӧ�����߶��Ը������Ϊ��Ӧ����ǽ����ͻ�ƿڡ�����Ȼ������ɫ���嵥��Ϊ����������DΪ������AΪ������ء���ͼ���յ�����Ϊ���������Ǻ����ᷴӦ�����ԣ��ڢۢܢ��ĸ���Ӧ�Ĺ�ͬ��Ϊ�ò�ͬ������������Ũ��������Ϊ��������Ԫ�صĻ��ϼ���-1�����ߵ�0�ۡ����ݢۢܢ�������Ӧ�з�Ӧ�����IJ��죨���·�Ӧ�����ȡ����ȴ��������ó�A��C��D���������ɴ�С��˳��ΪKMnO4>MnO2>O2��

��ϰ��ϵ�д�
 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д�
�����Ŀ
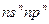 ��CԪ�صĵ�һ��������ͬ��Ԫ��������Ҹ���ͬ�����������ڵ�Ԫ�أ���
��CԪ�صĵ�һ��������ͬ��Ԫ��������Ҹ���ͬ�����������ڵ�Ԫ�أ���