��Ŀ����
���и���������ָ����Һ���ܴ����������
����ɫ��Һ�У�K+��Na+��MnO4-��SO42�� ��pH=11����Һ�У�CO32����Na+��NO3����AlO2-
�ۼ���Al�ܷų�H2����Һ�У�Cl����SO42����NO3����Mg2+
������ˮ�������c(OH��)=10��13mol��L��1����Һ�У�Na+��Ba2+��Cl����I��
������Һ���ܴ������棬����NaOH����ȼ�������ų����г������ɵ���Һ��
Ca2+��HCO3����NH4+��AlO2��
����ʹ��̪���ɫ����Һ��Na+��C1����S2����SO32��
A���٢ۢݡ��� B���ڢܢ� C���ڢܢޡ� D���ܢݢ�
C
����:����MnO4-Ϊ�Ϻ�ɫ���������⣬�ų�A������NH4++OH-NH3��+H2O,
HCO3��+ OH-=CO32- CO32-+Ca2+= CaCO3��,�������⡣���Ԣ٢���ȷ��ѡ��A��

��ϰ��ϵ�д�
�����Ŀ
 ��Na+��HSO
��Na+��HSO ��PO
��PO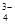 ��MnO
��MnO
 ��NO
��NO ��SO
��SO
 ��CO
��CO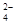 ��Cl
��Cl =0.1 mol/L����Һ�У�Na+��K+��SO
=0.1 mol/L����Һ�У�Na+��K+��SO