��Ŀ����
�����ȷ���ʽ˵����ȷ���ǣ�
A���к��Ȧ�H����57.3 kJ��mol��1,, ��2H��(aq)��![]() (aq)��Ba2��(aq)��2OH��(aq)===BaSO4(s)��2H2O(l)�� ��H����114.6 kJ��mol��1
(aq)��Ba2��(aq)��2OH��(aq)===BaSO4(s)��2H2O(l)�� ��H����114.6 kJ��mol��1
B����0.5 molN2��1.5 mol H2���ܱ���������NH3(g)������19.3 kJ������ʽΪ��
N2(g)+3H2(g) ![]() 2NH3(g) ��H=��38.6 kJ��mol��1
2NH3(g) ��H=��38.6 kJ��mol��1
C. ��״����H2(g)��F2(g) ===2HF(g) ��H����270kJ/mol��
D����������4NH3(g)��5O2(g) ===4NO(g)��6H2O(g) ��H����1025 kJ/mol
D

��ϰ��ϵ�д�
�����Ŀ
�����ȷ���ʽ˵����ȷ���ǣ�
A���к��Ȧ�H����57.3 kJ��mol��1,, ��2H��(aq)�� (aq)��Ba2��(aq)��2OH��(aq)===BaSO4(s)��2H2O(l)����H����114.6 kJ��mol��1 (aq)��Ba2��(aq)��2OH��(aq)===BaSO4(s)��2H2O(l)����H����114.6 kJ��mol��1 |
| B����0.5 molN2��1.5 mol H2���ܱ���������NH3(g)������19.3 kJ������ʽΪ�� N2(g)+3H2(g)  2NH3(g)��H="��38.6" kJ��mol��1 2NH3(g)��H="��38.6" kJ��mol��1 |
| C����״����H2(g)��F2(g) ==="2HF(g)" ��H����270kJ/mol�� |
| D����������4NH3(g)��5O2(g) ===4NO(g)��6H2O(g)��H����1025 kJ/mol |
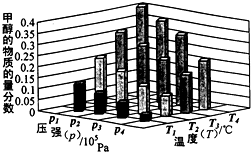 ��Դ����Լ���ҷ�չ���̵�����֮һ���״��������ѵȱ���Ϊ2 1���͵���ɫ��Դ����ҵ��������Ȼ��Ϊ��Ҫԭ���������̼��ˮ������һ���������Ʊ��ϳ�����CO��H2�������Ƴɼ״��������ѣ�
��Դ����Լ���ҷ�չ���̵�����֮һ���״��������ѵȱ���Ϊ2 1���͵���ɫ��Դ����ҵ��������Ȼ��Ϊ��Ҫԭ���������̼��ˮ������һ���������Ʊ��ϳ�����CO��H2�������Ƴɼ״��������ѣ�