��Ŀ����
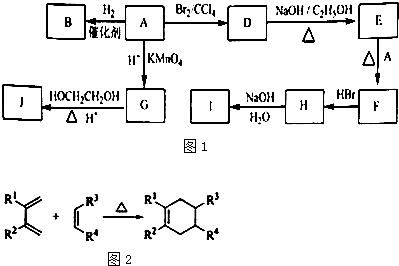
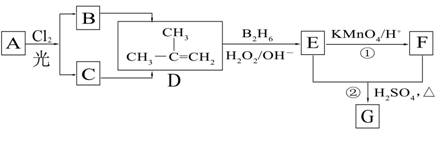
(12��)�л�������A��G������ת����ϵ��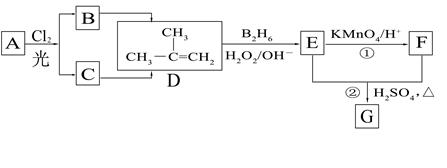
��֪��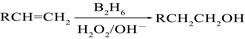 ��B2H6Ϊ�����飩
��B2H6Ϊ�����飩
����д���пհף�
��1��11.2L����״��������A�������г��ȼ�տ��Բ���88g CO2��45g H2O��A�ķ���ʽ�ǣ� ��
��2��B��C��Ϊһ�ȴ��������ǵĹ�ϵ�� ��
��3����Ӧ�ٵķ�Ӧ�����ǣ� ��
��4����Ӧ�ڵĻ�ѧ����ʽ�ǣ�
��
��5��������ͬ�����ŵ�F��ͬ���칹��Ľṹʽ�ǣ�
��
��6����һ�������£���D�ۺϵõ��ĸ߷��ӻ�����Ľṹ��ʽ�ǣ�
��
��ÿ��2�֣���12�֣���1��C4H10 ����2��ͬ���칹�塣��3��������Ӧ��
��4��(CH3)2CHCH2OH��(CH3)2CHCOOH
(CH3)2CHCOOCH2CH(CH3)2 ��H2O
��5��CH3CH2CH2COOH����6��
����

( 12��)������Ԫ�أ�����A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������ش����⡣
|
AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
|
BԪ��ԭ�ӵĺ���p��������s��������1 |
|
Cԭ�ӵĵ�һ�����ĵ����ֱܷ���: I1=738kJ/mol I2 = 1451 kJ/mol I3 = 7733kJ/mol I4 = 10540kJ/mol |
|
Dԭ�Ӻ�������p���ȫ������� |
|
EԪ�ص������������������IJ�Ϊ4 |
|
F��ǰ�������е縺����С��Ԫ�� |
|
G�����ڱ��ĵڰ��� |
��1����֪BA5 Ϊ���ӻ�������� �� ���������ɵģ��ѧ���ţ���
��2��B��̬ԭ����������ߵĵ��ӣ���������ڿռ��� ������ԭ�ӹ���� �Ρ�
��3��ijͬѧ����������Ϣ���ƶ�C��̬ԭ�ӵĺ�������Ų�Ϊ��
��ͬѧ�����ĵ����Ų�ͼΥ���� ��
��4��G�
�壬G3+�۵����Ų�ʽΪ
��GE3������Ϊ���壬�۵� ���е�
���е� ����
����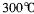 ����������������ˮ��Ҳ���������ѡ���ͪ���л��ܼ����ݴ��ж�GE3�ľ�������Ϊ________________��
����������������ˮ��Ҳ���������ѡ���ͪ���л��ܼ����ݴ��ж�GE3�ľ�������Ϊ________________��
��5��DE3 ����ԭ�ӵ��ӻ���ʽΪ ����ռ乹��Ϊ ��
��6��ǰ����������Fͬһ�������Ԫ�طֱ���EԪ���γɻ�����侧����۵��ɸߵ��͵�����˳��Ϊ��д��ѧʽ�� ��ԭ����
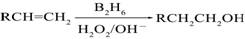 ��B2H6Ϊ�����飩
��B2H6Ϊ�����飩