��Ŀ����
( 12��)������Ԫ�أ�����A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������ش����⡣
|
AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
|
BԪ��ԭ�ӵĺ���p��������s��������1 |
|
Cԭ�ӵĵ�һ�����ĵ����ֱܷ���: I1=738kJ/mol I2 = 1451 kJ/mol I3 = 7733kJ/mol I4 = 10540kJ/mol |
|
Dԭ�Ӻ�������p���ȫ������� |
|
EԪ�ص������������������IJ�Ϊ4 |
|
F��ǰ�������е縺����С��Ԫ�� |
|
G�����ڱ��ĵڰ��� |
��1����֪BA5 Ϊ���ӻ�������� �� ���������ɵģ��ѧ���ţ���
��2��B��̬ԭ����������ߵĵ��ӣ���������ڿռ��� ������ԭ�ӹ���� �Ρ�
��3��ijͬѧ����������Ϣ���ƶ�C��̬ԭ�ӵĺ�������Ų�Ϊ��
��ͬѧ�����ĵ����Ų�ͼΥ���� ��
��4��G�
�壬G3+�۵����Ų�ʽΪ
��GE3������Ϊ���壬�۵�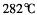 ���е�
���е�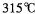 ����
����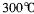 ����������������ˮ��Ҳ���������ѡ���ͪ���л��ܼ����ݴ��ж�GE3�ľ�������Ϊ________________��
����������������ˮ��Ҳ���������ѡ���ͪ���л��ܼ����ݴ��ж�GE3�ľ�������Ϊ________________��
��5��DE3 ����ԭ�ӵ��ӻ���ʽΪ ����ռ乹��Ϊ ��
��6��ǰ����������Fͬһ�������Ԫ�طֱ���EԪ���γɻ�����侧����۵��ɸߵ��͵�����˳��Ϊ��д��ѧʽ�� ��ԭ����
��12�֣���1��NH4+ H- ��1�֣���2�� 3 ���Ĵ��Σ���1�֣���2�֣�
��3������ԭ�� ��1�֣���4�� �ڢ� ��3s23p63d5 �����Ӿ��壨��1�֣���3�֣�
��5�� sp3�� ���� ����1�֣���2�֣�
��6��LiCl��NaCl��KCl��HCl��LiCl��NaCl��KCl��Ϊ���Ӿ��壬HClΪ���Ӿ��壬����HCl�۵���͡�����ΪLi+��Na+��K+�İ뾶��������LiCl��NaCl��KCl�ľ��������μ�С�����۵����ν��͡���1��+2�֣�
��������
�����������������ḻ��Ԫ����H������A����Ԫ�أ�BԪ��ԭ�ӵĺ���p��������s��������1�����Ը��ݹ���ԭ����֪��BӦ����N��CԪ�صĵ���������Զ���ڵڶ������ܣ�����C�ǵڢ�AԪ�أ�����ԭ��������֪��C��Mg��Dԭ�Ӻ�������p���ȫ�����������D��P��EԪ�ص������������������IJ�Ϊ4����ԭ����������15������E����Ԫ�أ�F��ǰ�������е縺����С��Ԫ�أ����ڽ�����Խǿ���縺��ԽС������F��K��G�����ڱ��ĵڰ��У������ڵ������ڣ�����G����Ԫ�ء�
��1������BA5 Ϊ���ӻ������������NH4+�� H-���������ɡ�
��2��B��̬ԭ����������ߵĵ�����2p3���ӣ���������ڿռ���3������ԭ�ӹ���ʷĴ��Ρ�
��3��1��ԭ�ӹ�����������2�����ӣ������������෴ ���������ԭ�������Ը��ݵ����Ų�ͼ��֪��Υ��������ԭ����
��4����λ�ڵڢ��壬���ݹ���ԭ����֪�����ӵļ۵����Ų�ʽΪ3s23p63d5�������Ȼ������۷е�ϵ͡����������ܽ��Կ�֪���û������γɵľ���Ӧ���Ƿ��Ӿ��塣
��5�����Ȼ���������ԭ��Pԭ�Ӻ��еŶԵ��Ӷ����ǣ�5��1��3����2��1�������������νṹ������sp3�ӻ���
��6������LiCl��NaCl��KCl��Ϊ���Ӿ��壬��HClΪ���Ӿ��壬����HCl�۵���͡�����ΪLi+��Na+��K+�İ뾶��������LiCl��NaCl��KCl�ľ��������μ�С�������۵����ν��ͣ�����ȷ��˳����LiCl��NaCl��KCl��HCl��
���㣺����Ԫ�����ڱ��Ľṹ�������ܡ��縺�ԡ���������Ų���������й��жϵ�
�����������ԡ����ڱ���Ԫ�ص��ƶϡ�Ϊ���壬����ѧ����Ԫ�����ڱ�����Ϥ�̶ȼ���Ա��и�Ԫ�����ʺ���Ӧԭ�ӽṹ�������Եݱ���ɵ���ʶ�����ճ̶ȡ�������ѧ�������ʽṹ�����ʹ�ϵ�Լ�����Ԫ�������ɽ�����廯ѧ�����������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�(15��)
��������Ԫ�أ�����A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⣮
| AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
| BԪ��ԭ�ӵĺ���p��������s��������1 |
| Cԭ�ӵĵ�һ�����ĵ����ֱܷ���: I1=738kJ/mol I2 = 1451 kJ/mol I3 = 7733kJ/mol I4 = 10540kJ/mol |
| Dԭ�Ӻ�������p���ȫ������� |
| EԪ�ص������������������IJ�Ϊ4 |
| F��ǰ�������е縺����С��Ԫ�� |
| G�����ڱ��ĵ����� |
����֪BA5 Ϊ���ӻ����д�������ʽ ��
��B��̬ԭ����������ߵĵ��ӣ���������ڿռ��� ������ԭ�ӹ���� ��
 ��ijͬѧ����������Ϣ���ƶ�C��̬ԭ�ӵĺ�������Ų�Ϊ��
��ijͬѧ����������Ϣ���ƶ�C��̬ԭ�ӵĺ�������Ų�Ϊ��
��ͬѧ�����ĵ����Ų�ͼΥ���� ��
��Gλ�� �� �����۵����Ų�ʽΪ ��
��DE3 ����ԭ�ӵ��ӻ���ʽΪ ���ü۲���ӶԻ��������Ʋ���ռ乹��
Ϊ ��
�ʼ���FԪ�صķ����� ������ԭ�ӽṹ��֪ʶ���Ͳ����������ԭ���� ��
����ij���ʾ�����ԭ�ӵĶѻ���ʽ����ͼ����ʾ���侧����������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ����ͼ����ʾ�����и�ԭ�ӵ���λ��Ϊ ���õ��ʾ�����ԭ�ӵĶѻ���ʽΪ���ֻ���ģʽ�е� ��
(15��)
��������Ԫ�أ�����A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⣮
|
AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
|
BԪ��ԭ�ӵĺ���p��������s��������1 |
|
Cԭ�ӵĵ�һ�����ĵ����ֱܷ���: I1=738kJ/mol I2 = 1451 kJ/mol I3 = 7733kJ/mol I4 = 10540kJ/mol |
|
Dԭ�Ӻ�������p���ȫ������� |
|
EԪ�ص������������������IJ�Ϊ4 |
|
F��ǰ�������е縺����С��Ԫ�� |
|
G�����ڱ��ĵ����� |
����֪BA5 Ϊ���ӻ����д�������ʽ ��
��B��̬ԭ����������ߵĵ��ӣ���������ڿռ��� ������ԭ�ӹ���� ��
 ��ijͬѧ����������Ϣ���ƶ�C��̬ԭ�ӵĺ�������Ų�Ϊ��
��ijͬѧ����������Ϣ���ƶ�C��̬ԭ�ӵĺ�������Ų�Ϊ��
��ͬѧ�����ĵ����Ų�ͼΥ���� ��
��Gλ�� �� �����۵����Ų�ʽΪ ��
��DE3 ����ԭ�ӵ��ӻ���ʽΪ ���ü۲���ӶԻ��������Ʋ���ռ乹��
Ϊ ��
�ʼ���FԪ�صķ����� ������ԭ�ӽṹ��֪ʶ���Ͳ����������ԭ���� ��
����ij���ʾ�����ԭ�ӵĶѻ���ʽ����ͼ����ʾ���侧����������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ����ͼ����ʾ�����и�ԭ�ӵ���λ��Ϊ ���õ��ʾ�����ԭ�ӵĶѻ���ʽΪ���ֻ���ģʽ�е� ��

������Ԫ�أ�����A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������ش����⡣
| AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
| BԪ��ԭ�ӵĺ���p��������s��������1 |
| Cԭ�ӵĵ�һ�����ĵ����ֱܷ���: I1=738kJ/mol I2 = 1451 kJ/mol I3 = 7733kJ/mol I4 = 10540kJ/mol |
| Dԭ�Ӻ�������p���ȫ������� |
| EԪ�ص������������������IJ�Ϊ4 |
| F��ǰ�������е縺����С��Ԫ�� |
| G�����ڱ��ĵڰ��� |
��1����֪BA5 Ϊ���ӻ�������� �� ���������ɵģ��ѧ���ţ���
��2��B��̬ԭ����������ߵĵ��ӣ���������ڿռ��� ������ԭ�ӹ���� �Ρ�
![]() ��3��ijͬѧ����������Ϣ���ƶ�C��̬ԭ�ӵĺ�������Ų�Ϊ��
��3��ijͬѧ����������Ϣ���ƶ�C��̬ԭ�ӵĺ�������Ų�Ϊ��
��ͬѧ�����ĵ����Ų�ͼΥ���� ��
��4��Gλ�� �壬G3+�۵����Ų�ʽΪ ��GE3������Ϊ���壬�۵�![]() ���е�
���е�![]() ����
����![]() ����������������ˮ��Ҳ���������ѡ���ͪ���л��ܼ����ݴ��ж�GE3�ľ�������Ϊ________________��-ks5u
����������������ˮ��Ҳ���������ѡ���ͪ���л��ܼ����ݴ��ж�GE3�ľ�������Ϊ________________��-ks5u
��5��DE3 ����ԭ�ӵ��ӻ���ʽΪ ����ռ乹��Ϊ ��
��6��ǰ����������Fͬһ�������Ԫ�طֱ���EԪ���γɻ�����侧����۵��ɸߵ��͵�����˳��Ϊ��д��ѧʽ�� ��ԭ����
��
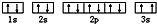 ��ͬѧ�����ĵ����Ų�ͼΥ����
��ͬѧ�����ĵ����Ų�ͼΥ����