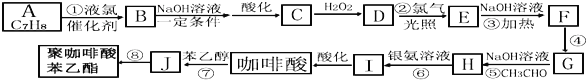
��Ŀ����
����Ŀ��þ���Ͻ�������õ�ǿ�ȡ����ԡ������ĵ�������Ժ͵����ԣ�ʹ֮��Ϊ�ʼDZ����Ժ��ᱡ�ֻ�����ѡ��Dz��ϡ�Ϊ�ⶨij�ֻ����þ���Ͻ���þ�����ĺ��������þ����ijʵ��С���������ʵ�鷽����
�ٳ�ȥ�ֻ���ǵ�Ϳ�㣬������һС�飬��ȡ��������
����װ��������������ԣ�������ϡ�����ܽ�Ͻ�
�۲�������������������������
�������������̣����ܽ�Ͻ�����Һ�л��շ���þ����
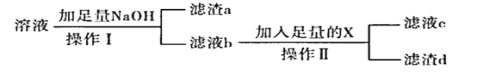
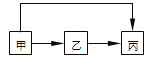
��1����������ͼ�������������һ��ʵ��װ�ã�ÿ������ֻ��ʹ��һ�Σ��ɲ�ѡ�ã��������������ӵ��Ⱥ�˳����Сд��ĸ��ʾ��_______

��2�����˲ⶨ�������������⣬��Ӧ�ⶨ_______��
��3�����������У�����I��Ҫ�IJ����������ձ�����������_______��Ϊ��֤���ղ�Ʒ�Ĵ��ȣ�����I��Ӧ_______��
��4����Һb��������_______���ѧʽ�����Լ�X��_______��
��5��������a�м������ᣬ������_______���ѧʽ�������Ӿ����л�ý�����Ӧ���еIJ�����_______��
��6�����ӳ�ȥͿ����ֻ�����ȡ��Ʒmg�������������ò���������nL��ʵ�������µ�����Ħ�����ΪVmL/mol����Ͻ���Mg������Ϊ________g��
���𰸡�afed �¶ȡ�ѹǿ ©�� ϴ�ӳ��� NaAlO2 CO2 MgCl2��H2O ���ڵ�� ![]()
��������
(1)�ֻ���ǵ�Ϳ�㣬��һС�飬������ϡ�����ܽ�Ͻ𣬲���������ͨ����ˮ�����������������װ�õ�����˳��Ϊafed��
(2)�ڼ�������У���Ҫ���������ת��Ϊ���ʵ������ʳ��˲ⶨ�������������⣬��Ҫ�����ڸ�����µ��¶�ѹǿ���Ӷ�ȷ������Ħ��������ٽ�����ؼ��㣻
(3)���������У�����I������Ҫ�IJ����������ձ�����������©����Ϊ��֤���ղ�Ʒ�Ĵ��ȣ�����I��Ӧϴ�ӳ�����
(4)���������������Ʒ�Ӧ��������ΪNaAlO2��������Һb��������ΪNaAlO2��NaAlO2��CO2��Ӧ������������������
(5)����aΪ������þ���������ᣬ��Ӧ���ɵIJ���ΪMgCl2��H2O��������Һ�л������ˮ���壬Ӧ���еIJ�������HCl�������������ᾧ�����գ�þ�ǻ��ý�����Ҫ�õ�������Ҫ���ڵ��MgCl2���壻
(6)�ӳ�ȥͿ����ֻ�����ȡ��Ʒmg�������������ò���������nL��ʵ�������µ�����Ħ�����ΪVmL/mol������뷴Ӧ��þ������Ϊxg������뷴Ӧ���������ʵ���Ϊ(m-x)g������Mg��2HCl=MgCl2��H2����2Al��6HCl=2AlCl3��3H2�����ɵ�![]() ���ɽ�ò��뷴Ӧ��þ������
���ɽ�ò��뷴Ӧ��þ������![]() ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�