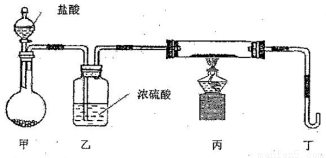
��Ŀ����
���������������
��Ħ���ǹ��ʵ�λ�����߸�����������֮һ����1mol�κ����ʶ�����Լ6.02��1023��ԭ�ӣ������ʵ���Ũ��Ϊ0.1 mol/L��AlCl3��Һ�У�Cl-�ĸ�����0.3NA������ԭ�ӵ�Ħ��������1 g����HCl��Ħ����������1 mol HCl���ӵ���������1mol SO2�������22.4L��
A���٢ڢ� B���ڢۢ� C���ڢܢݢ� D��ȫ��
�¶�ΪTʱ����4.0 L�����ܱ������г���2.0 mol PCl5��������Ӧ:
PCl5��g�� PCl3��g����Cl2��g������Ӧʱ�䣨t����������������ѹǿ��p�������ݼ��±���
PCl3��g����Cl2��g������Ӧʱ�䣨t����������������ѹǿ��p�������ݼ��±���
t/s | 0 | 50 | 150 | 250 | 350 |
��ѹǿp/100kPa | 100 | 116 | 119 | 120 | 120 |
��1������ѹǿp����ʼѹǿp0���㷴Ӧ��PCl5��ת���ʦ���PCl5���ı���ʽΪ��ƽ��ʱPCl5��ת����Ϊ����?
��2����Ӧ��ǰ50 s��ƽ������v��PCl3��Ϊ���٣�
��3�����¶��� ��ƽ�ⳣ��Ϊ���٣�
��ƽ�ⳣ��Ϊ���٣�
A����500 mL����ƿ������500 mL�ռ���Һ |
B������Һ�ܣ����ʽ�ζ��ܣ���ȡ50.00 mL�ռ���Һ����ƿ�в��Ӽ��μ���ָʾ�� |
C������ƽ��ȷ��ȡ�ռ���Ʒm g�����ձ��м�����ˮ�ܽ� |
D�������ʵ���Ũ��Ϊc mol/L�ı�H2SO4��Һװ����ʽ�ζ��ܣ�����Һ�棬���¿�ʼ�̶�V1mL |
E������ƿ�µ�һ�Ű�ֽ���ζ����յ㣬��¼�յ�̶�ΪV2mL ������������⣺
��1����ȷ�IJ��������ǣ���д��ĸ��________��________��________��D��________.
��2���ζ��ܶ���Ӧע��_______________________.
��3����������ƿ�µ�һ�Ű�ֽ��������___________________.
��4������D��Һ��Ӧ������________________________�����첿��Ӧ____________��
��5���ζ����յ�ʱ��ƿ����Һ��pHԼΪ___________���յ�ʱ����ɫ�仯��________��
��6������ʽ�ζ���û�ñ�H2SO4��ϴ����Բⶨ����к�Ӱ��________���ƫ�ߡ�����ƫ�͡�����Ӱ�족��������������ȷ����
��7�����ռ���Ʒ�Ĵ��ȼ���ʽ��__________________��



 ��Fe2��֮���γɵĻ�ѧ�����������Ӽ�
��Fe2��֮���γɵĻ�ѧ�����������Ӽ� �������н���1��̼ԭ�Ӳ�ȡsp3�ӻ�
�������н���1��̼ԭ�Ӳ�ȡsp3�ӻ� CO��g��+Cl2��g�� ��H>0������Ӧ�ﵽƽ��ʱ�����д�ʩ�����COCl2ת���ʵ���
CO��g��+Cl2��g�� ��H>0������Ӧ�ﵽƽ��ʱ�����д�ʩ�����COCl2ת���ʵ���