��Ŀ����
����Ŀ��ͨ���Ѳ�1 molij��ѧ�������յ��������ɸû�ѧ���ļ��ܣ���֪���ֻ�ѧ���ļ������£�
��ѧ�� | N��H | N��N | O=O | N��N | O��H |
����(kJ��mol��1) | 386 | 167 | 498 | 946 | 460 |
��1���������۷ɴ��ij������������(N2H4����̬)Ϊȼ�ϣ�����������(��̬)��ȼ�գ�����N2(��̬)��H2O(Һ̬)��1 mol����ȫȼ��ʱ�ų�������Ϊ________��
��2����-����ȼ�ϵ����һ�ּ���ȼ�ϵ�أ��������Һ��20%��30%��KOH ��Һ����-����ȼ�ϵ�طŵ�ʱ�������ĵ缫��Ӧʽ��______________________________________��
��3����֪N60������ÿ��Nԭ�Ӿ��Ե��������������ԭ�ӣ���N60���ӽṹ��ÿ����ԭ�����γ�8�����ӵ��ȶ��ṹ�����Ʋ�1��N60�Ľṹ����________��N��N����
���𰸡���1��577 kJ��2��O2��4e����2H2O=4OH����3��90
��������
���������
��1��N2H4ȼ�յĻ�ѧ����ʽΪN2H4��g��+O2��g���TN2��g��+2H2O��g������H=��Ӧ����ܼ���-��������ܼ���=167 kJ��mol-1+386 kJ��mol-1��4+498 kJ��mol-1-946 kJ��mol-1-460 kJ��mol-1��4=-577 kJ��mol-1��N2H4ȼ�յ��Ȼ�ѧ����ʽΪN2H4��g��+O2��g���TN2��g��+2H2O��g����H=-577 kJ��mol-1������1mol����ȫȼ��ʱ�ų�������Ϊ577 kJ���ʴ�Ϊ��577kJ��
��2���£�����ȼ�ϵ���У�O2�������ŵ磬����OH����
��3��N60������ÿ��Nԭ�Ӿ��Ե��������������ԭ�ӣ�������Ϊ������ԭ�ӹ��ã�1��N60�Ľṹ����N��N��������![]() ��
��

 ��ʱ�ƿ�������ϰϵ�д�
��ʱ�ƿ�������ϰϵ�д� һ��һ��һ��ͨϵ�д�
һ��һ��һ��ͨϵ�д� �㽭֮��ѧҵˮƽ����ϵ�д�
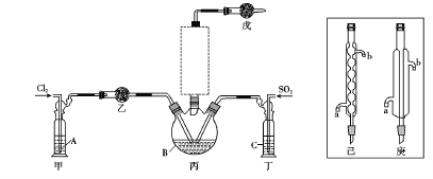
�㽭֮��ѧҵˮƽ����ϵ�д�����Ŀ����X��Y��Z��T��U���ֶ�����Ԫ�ء�X��Y��Z��Ԫ�������ڱ��е�λ��������ʾ����Ԫ�ص�ԭ������֮����41��X��T�ĵ����ڲ�ͬ�����·�Ӧ����������T2X(��ɫ����)��T2X2(����ɫ����)���ֻ����U������Z������ȼ��ʱ������ɫ���棬�������ˮ��Һ��ʹʯ����Һ��졣
X | |
Y | Z |
��1����Ԫ�صķ����ǣ�Z________��T________
��2��Yԭ�ӵĽṹʾ��ͼΪ____________________,U2X�ĵ���ʽ
��3��YX2��U2Y��Ӧ�Ļ�ѧ����ʽΪ____________________________��������������____________����������Ԫ����____________��