��Ŀ����
����Ŀ��ij�л�������A����Է�������С��200����������֪������̼����������Ϊ64.52%�������������Ϊ9.68%������Ϊ����A�������ԣ��Ƿ������е���Ч�ɷ֣�37.2 mg A ��Ҫ20.0 mL 0.010 0mol��L-1 ��������ˮ��Һ���ζ��ﵽ�յ㡣
��1���л�������A����Է���������_______________________��
��2���û�����ķ���ʽ��_______________________��
��3���÷����к���__________���Ȼ����ƶϹ��̣�__________________________________��
���𰸡�186C10H18O31n(C10H18O3)=![]() =2��10-4mol��n(NaOH)= 2��10-4mol��A��NaOH�����ʵ���֮��1:1��Ӧ�����Ը��л�����ӽṹ�к���һ���Ȼ���
=2��10-4mol��n(NaOH)= 2��10-4mol��A��NaOH�����ʵ���֮��1:1��Ӧ�����Ը��л�����ӽṹ�к���һ���Ȼ���
��������
��1�����л�������C��H��O����Ԫ����ɣ����������������Ϊ(1��64.52%��6.68%)=25.8%��C��H��O��ԭ�Ӹ�����Ϊ64.52%/12��9.68%/1��25.8%/16=10��8��3�����л�������ʽΪC10H8O3����ΪA����Է�������С��200�������(C10H8O3)n����Է�������С��200����n=1�����л������ʽΪC10H8O2����Է�������Ϊ186����2�����ݣ�1���ķ��������л������ʽΪC10H8O2����3��A�������ԣ�˵�������Ȼ�������A�ķ���ʽ��1mol���л��ﺬ��3molO����˸��л����к���1���Ȼ�������n(C10H18O3)=![]() =2��10-4mol��n(NaOH)= 2��10-4mol��A��NaOH�����ʵ���֮��1:1��Ӧ�����Ը��л�����ӽṹ�к���һ���Ȼ���
=2��10-4mol��n(NaOH)= 2��10-4mol��A��NaOH�����ʵ���֮��1:1��Ӧ�����Ը��л�����ӽṹ�к���һ���Ȼ���

����Ŀ����������ʵ���������ý�����ȷ����( )
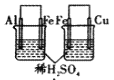
ʵ�� | ʵ������ | ���� | |
A |
| ���ձ��������������ݣ��ұ��ձ���ͭ���������� | �����ԣ�Al3+>Fe2+>Cu2+ |
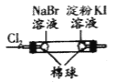
B |
| �������Ϊ��ɫ���ұ�����Ϊ��ɫ | �����ԣ�Cl2>Br2>I2 |
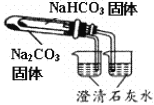
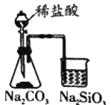
C |
| ����ձ��������Ա仯���ұ��ձ��г���ʯ��ˮ����� | ���ȶ��ԣ�Na2CO3>NaHCO3 |
D |
| ��ƿ��������������ձ���Һ������ | �ǽ����ԣ�Cl>C>Si |
A. AB. BC. CD. D