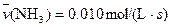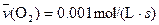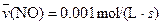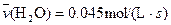��Ŀ����
 ����ҵ�Ϻϳɰ�����һ�������½������·�Ӧ��N2��g��+3H2��g��
����ҵ�Ϻϳɰ�����һ�������½������·�Ӧ��N2��g��+3H2��g�� 2NH3��g�����䲿�ֹ����������£�
2NH3��g�����䲿�ֹ����������£�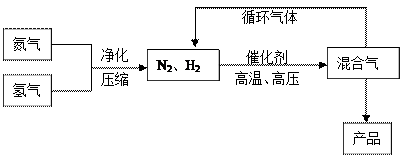 |






 �ش��������⣺
�ش��������⣺ ����֪��N2(g)+O2(g) =2NO(g)����H=180.5kJ/mol
����֪��N2(g)+O2(g) =2NO(g)����H=180.5kJ/mol 4NH3(g)+5O2(g)=4NO(g)+6H2O(g) ����H=-905kJ/mol
4NH3(g)+5O2(g)=4NO(g)+6H2O(g) ����H=-905kJ/mol 2H2(g)+O2(g)=2H2O(g) ����H=-483.6kJ/mol
2H2(g)+O2(g)=2H2O(g) ����H=-483.6kJ/mol ��N2��g��+3H2��g��
��N2��g��+3H2��g�� 2NH3��g���ġ�H=_________________��
2NH3��g���ġ�H=_________________��


 ��2������ó������İ�ˮ��pH=a��������ͬ���������ʱ����Һ�����ԣ���������pH_________14��a����ʱc(NH4+)________c(Cl-)(����ڡ���С�ڡ����ڡ�)��
��2������ó������İ�ˮ��pH=a��������ͬ���������ʱ����Һ�����ԣ���������pH_________14��a����ʱc(NH4+)________c(Cl-)(����ڡ���С�ڡ����ڡ�)����1���£�N2H4���ֳ���������һ�ֿ�ȼ�Ե�Һ�壬���������ȼ�ϡ���֪��101kPa
ʱ��16.0gN2H4����������ȫȼ�����ɵ������ų�����312kJ��
 д����ʾN2H4ȼ���ȵ��Ȼ�ѧ����ʽ�� ��
д����ʾN2H4ȼ���ȵ��Ȼ�ѧ����ʽ�� ����2���¡�����ȼ�ϵ����һ�ּ���ȼ�ϵ�أ��������Һ��20%��30%��KOH��Һ���¡�����ȼ�ϵ�طŵ�ʱ��
|
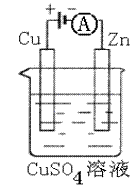
��3����ͼ��һ���绯ѧ����ʾ��ͼ��
��пƬ�Ϸ����ĵ缫��Ӧ�� ��
�ڼ���ʹ���¡�����ȼ�ϵ����Ϊ�������еĵ�Դ��
ͭƬ�������仯128g������һ����ȼ�ϵ������
�����ı�״���µĿ��� L
����������������������Ϊ20%��
����

 ��1����92.4kJ/mol
��1����92.4kJ/mol��2����
 ����
������1�� N2H4(l)+O2(g)=N2(g)+H2O(l);��H="-624kJ/mol "
��2�� O2+4e-+2H2O=4OH- �� N2H4-4e-+4OH-=N2��+4H2O
��3�� Cu2+ + 2e -=" Cu " �� 112
�����ݸ�˹���ɿɵã��Ȼ�ѧ��Ӧ����ʽN2��g��+3H2��g��
 2NH3��g���ġ�H=180.5kJ/mol+[-��-905kJ/mol��1/2��]+[-483.6kJ/mol��3/2]=��92.4kJ/mol
2NH3��g���ġ�H=180.5kJ/mol+[-��-905kJ/mol��1/2��]+[-483.6kJ/mol��3/2]=��92.4kJ/mol��2���������pH=14��aʱ�����������İ�ˮ�����кͷ�Ӧ����Һһ���ʼ��ԣ���ˮ��������Ҫ��ʣ������Ҫ������кͷ�Ӧ���Һ�������ԣ��������Ũ��Ҫ��һ�㣬����Һ��PHС��14��a�����ݵ���غ��֪��c(H+)+c(NH4+)=c(OH-)+c(Cl-)������Һ�����ԣ���c(H+)=c(OH-)����c(Cl-)=c(NH4+)��
����1����16.0gN2H4��ȫȼ�շų�����312kJ�������1mol����ȫȼ�����ų�������Ϊ624k���ʱ�ʾ����ȼ���ȵ��Ȼ�ѧ����ʽΪN2H4(l)+O2(g)=N2(g)+H2O(l);��H="-624kJ/mol"
��2�����¡�����ȼ�ϵ����һ�ּ���ȼ�ϵ�ؿ�֪��������ӦΪ O2+4e-+2H2O=4OH-��������Ӧ�����ܵ缫��Ӧʽ��ȥ������Ӧʽ�������غ�ʱ�����ɵø�����ӦʽΪ N2H4-4e-+4OH-=N2��+4H2O ��
��3��ͭ�������仯Ϊ128g,��Cu-2e-=Cu2+,��֪�ڵ�·��ͨ���ĵ���Ϊ128g/64g.mol-1��2=4mole-,���ݵ����غ㣬�ֵ����ɷ�Ӧʽ N2H4(l)+O2(g)=N2(g)+H2O(l)�ṩ���ʿɵ�����1molO2(g),����Ϊ5V��O2��=5��22.4L=112L��

��ϰ��ϵ�д�
�����Ŀ


 2HI(g)��H��0,���ﵽƽ�⡣HI���������w(HI)��ʱ��t�仯��ͼ����(��)��ʾ��
2HI(g)��H��0,���ﵽƽ�⡣HI���������w(HI)��ʱ��t�仯��ͼ����(��)��ʾ��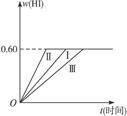
 2AB(g)�ﵽ�ȵı�־�ǣ� ��
2AB(g)�ﵽ�ȵı�־�ǣ� �� 2NH3��g��;��H=-92��4 kJ��mol-1��
2NH3��g��;��H=-92��4 kJ��mol-1��

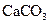 �T�T
�T�T 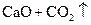
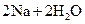 �T�T
�T�T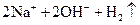
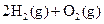 �T�T
�T�T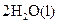
 4NO(g)+6H2O(g)��10L�ܱ������н��У�����Ӻ�ˮ���������ʵ���������0.45mol����˷�Ӧ��ƽ������
4NO(g)+6H2O(g)��10L�ܱ������н��У�����Ӻ�ˮ���������ʵ���������0.45mol����˷�Ӧ��ƽ������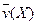 ����Ӧ����������ʻ������������ʣ��ɱ�ʾΪ�� ��
����Ӧ����������ʻ������������ʣ��ɱ�ʾΪ�� ��