��Ŀ����
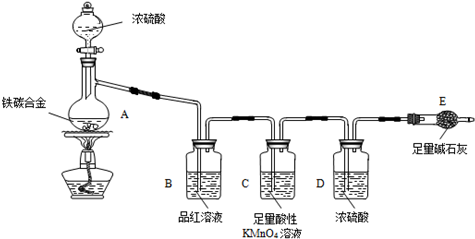
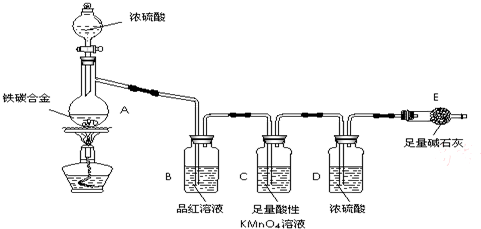
����ij��̼�Ͻ�����̼���ֵ��ʵĻ�����ij��ѧ��ȤС��Ϊ�˲ⶨ��̼�Ͻ�������������������̽��Ũ�����ijЩ���ʣ��������ͼ��ʾ��ʵ��װ�ã��г�������ʡ�ԣ���ʵ�鷽������ʵ��̽����

I.�ⶨ��������������
��1���������װ�������Ե�һ�ַ����ǣ��رշ�Һ©���Ļ�������Eװ�ú�������һ��
���ܣ�Ȼ��________________________________________����֤��װ�õ����������á�
��2������E������������a g��̼�Ͻ���Ʒ����װ��A�У��ټ���������Ũ���ᣬ��A�в����ݳ�����ʱ��ֹͣ���ȣ�����E�����أ�E����bg����̼�Ͻ���������������Ϊ_____________________________________________��д����ʽ����
��3��װ��C�з�Ӧ�����ӷ���ʽΪ________________________________________��
��4����ͬѧ��Ϊ�����ݴ�ʵ���õ����ݣ�����Ͻ������������������ܻ�ƫ�ͣ�ԭ���ǿ�����CO2��H2O����E��ʹb��������Ϊ�Ľ��ķ�����____________________________________.
��5����ͬѧ��Ϊ����ʹ��ͬѧ��Ϊ��ƫ��õ��Ľ������ݴ�ʵ���úϽ�������������
��Ҳ���ܻ�ƫ�롣����Ϊ���е�ԭ����_________________________________________��
��.̽��Ũ�����ijЩ���ʣ�
��6����A�еμ������Ĺ����ᣬδ��ȼ�ƾ���ǰ��A��B��������������ԭ���ǣ�
_____________________________________________________________________��
��7����A�й��廻Ϊ����ͭ����Ũ���ᷢ����Ӧ�Ļ�ѧ����ʽ��__________________
_______________________����Ӧ��Ũ������ֳ���������______________________��
��1���ѵ��ܷ���ʢˮ��ˮ���У�����ƿ��������ܿ������ݲ�����ֹͣ���Ⱥܿڲ���һ���ȶ���ˮ������2�֣�
��2��(11a-3b)/11a��100�G��2�֣�
��3��2MnO4-+5SO2+2H2O=2Mn2++5SO42-+4H+��2�֣�
��4����װ��E�ĺ��������һ����E��ͬ��װ�á���2�֣�
��5����Ӧ������CO2����δ����ȫ�ŵ�װ��E�У�����bƫ�͡�
��6�������£�����Ũ����ۻ���̼����Ũ���ᷴӦ����2�֣�
��7�� 2H2SO4(Ũ)+Cu CuSO2+
SO2��+2H2O; ��2�֣� ���Ժ�ǿ�����ԣ�2�֣�
CuSO2+
SO2��+2H2O; ��2�֣� ���Ժ�ǿ�����ԣ�2�֣�
��������
�����������1�����ȷ������ܷ�װ�õ������ԡ�
��2��E����bg,��CO2�����������Լ���̼������3b/11g,�������� (a-3b/11)g, ������������Ϊ(11a-3b)/11a��100�G.
��3��װ��C����K MnO4��Һ����ǿ������,�ܺ��л�ԭ�Ե�SO2��Ӧ�����ӷ���ʽΪ2MnO4-+5SO2+2H2O=2Mn2++5SO42-+4H+��
��4��Ϊ��ֹ������CO2��H2O����E�ܣ�������E�ĺ��������һ����E��ͬ��װ�ã����տ�����CO2��H2O��
��5����Ӧ������װ���ڻ������ɵ����������
��6�� �����£�����Ũ����ۻ���̼����Ũ���ᷴӦ��
��7�� ͭ��Ũ������ȵ������·�����Ӧ������CuSO2�� SO2��
���㣺Ũ��������ʣ�ͨ��ʵ�����ⶨ���ʵĴ��ȡ�

 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д� �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д�