��Ŀ����
A��B��C��D��E������Һ�����ʷֱ���HCl��CH3COOH��NaOH��NH3?H2O��Na2CO3�е�һ�֣������½�������ʵ�飺
��һ�����pH=10��A��Һ�ֱ���0.01mol?L-1x��B��Һ��0.01mol?L-1y��C��Һ��ַ�Ӧ������ʱ��x��y��С��ϵΪ��y��x��
��Ũ�Ⱦ�Ϊ0.1mol?L-1D��C��Һ�������ϣ���Һ�����ԣ�
��Ũ�Ⱦ�Ϊ0.1mol?L-1A��E��Һ��pH��A��E
��ش��������⣺
��1��B��______��Һ�������ʵĻ�ѧʽ����������______
��2����ˮϡ��0.1mol?L-1Dʱ����Һ������ˮ�������Ӷ���С����______����д��ţ�
��c��D��/c��OH-������c��OH-��/c��H+������c��H+����c��OH-���ij˻���OH-�����ʵ���
��3����C��Һ��εμӵ�A��Һ�У����������μӵ�A��C�����ʵ���ʱ����Һ�����������ӵ����ʵľ�Ũ����С�����˳����______��
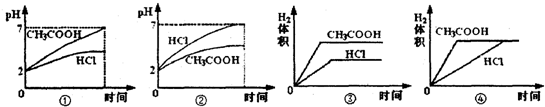
��4���������Ϊ1L��pH������2������ʹ�����Һ�зֱ�Ͷ��0.23gNa������ͼ�бȽϷ��Ϸ�Ӧ��ʵ��������______��
��һ�����pH=10��A��Һ�ֱ���0.01mol?L-1x��B��Һ��0.01mol?L-1y��C��Һ��ַ�Ӧ������ʱ��x��y��С��ϵΪ��y��x��
��Ũ�Ⱦ�Ϊ0.1mol?L-1D��C��Һ�������ϣ���Һ�����ԣ�
��Ũ�Ⱦ�Ϊ0.1mol?L-1A��E��Һ��pH��A��E
��ش��������⣺
��1��B��______��Һ�������ʵĻ�ѧʽ����������______
��2����ˮϡ��0.1mol?L-1Dʱ����Һ������ˮ�������Ӷ���С����______����д��ţ�
��c��D��/c��OH-������c��OH-��/c��H+������c��H+����c��OH-���ij˻���OH-�����ʵ���
��3����C��Һ��εμӵ�A��Һ�У����������μӵ�A��C�����ʵ���ʱ����Һ�����������ӵ����ʵľ�Ũ����С�����˳����______��
��4���������Ϊ1L��pH������2������ʹ�����Һ�зֱ�Ͷ��0.23gNa������ͼ�бȽϷ��Ϸ�Ӧ��ʵ��������______��

�Ƚ���Һ��Ϊ���ࣺ������ҺΪ��HCl��CH3COOH��������Һ��NaOH��NH3?H2O��Na2CO3��
���ݢ��֪AΪ������Һ����B��CΪ������Һ����B��CΪ��HCl��CH3COOH��
�ֱ���0.01mol?L-1x��B��Һ��0.01mol?L-1y��C��Һ��ַ�Ӧ������ʱ��x��y��С��ϵΪ��y��x��C�������٣�˵��B������C���Ա���������B��CH3COOH��CΪHCl��
�ɢ��֪��Ũ�Ⱦ�Ϊ0.1mol?L-1D��C��Һ�������ϣ���Һ�����ԣ�Ӧ����ǿ�������Σ���CΪHCl����DΪNH3?H2O��
�ٸ��ݢ��֪��DΪ��ˮ�������ݢ�Ũ�Ⱦ�Ϊ0.1mol?L-1 A��E��Һ��pH��A��E�������ƶ�AΪNa2CO3��EΪNaOH��
��1���ɢ��֪���кͼ�������Aֻ������ʹ��ᣬ�����ʵ�����A�ֱ�������ʵ���Ũ��B��C��ϳ����ԣ�C�������٣�˵��B������C���Ա���������B�Ǵ��ᣬ
�ʴ�Ϊ��CH3COOH���кͼ�������Aֻ������ʹ��ᣬ�����ʵ�����A�ֱ�������ʵ���Ũ��B��C��ϳ����ԣ�C�������٣�˵��B������C���Ա���������B�Ǵ��
��2����ˮϡ��0.1mol?L-1 NH3?H2Oʱ���ٽ�NH3?H2O�ĵ��룬��OH-�����ʵ�������NH3?H2O���ʵ���Ũ�ȼ�С���������������OH-Ũ�ȷ�����С����c��H+������
��٢ڼ�С�������¶Ȳ��䣬�۲��䣬������
�ʴ�Ϊ���٢ڣ�
��3����HCl��Һ��εμӵ�Na2CO3��Һ�У����������μӵ�HCl��Na2CO3�����ʵ���ʱ��ǡ������NaHCO3������HCO3-����ˮ��ʼ��ԣ���c��OH-����c��HCO3-����c��Cl-������ˮ����ڵ��룬��c��CO32-����c��OH-��������c��CO32-����c��OH-����c��HCO3-����c��Cl-����
�ʴ�Ϊ��c��CO32-����c��OH-����c��HCO3-����c��Cl-����
��4��pH������2������ʹ�����Һ�зֱ�Ͷ��0.23gNa���ƾ���ȫ��Ӧ��������ȫ��Ӧ������ʣ�࣬���������������ͬ�������Ϊ���ᣬ�����Ũ�ȴ�Ӧ���ʽϴ�HCl��pH�仯�ϴ�����ȷ��ͼ��Ϊ�ڢܣ�
�ʴ�Ϊ���ڢܣ�
���ݢ��֪AΪ������Һ����B��CΪ������Һ����B��CΪ��HCl��CH3COOH��
�ֱ���0.01mol?L-1x��B��Һ��0.01mol?L-1y��C��Һ��ַ�Ӧ������ʱ��x��y��С��ϵΪ��y��x��C�������٣�˵��B������C���Ա���������B��CH3COOH��CΪHCl��
�ɢ��֪��Ũ�Ⱦ�Ϊ0.1mol?L-1D��C��Һ�������ϣ���Һ�����ԣ�Ӧ����ǿ�������Σ���CΪHCl����DΪNH3?H2O��
�ٸ��ݢ��֪��DΪ��ˮ�������ݢ�Ũ�Ⱦ�Ϊ0.1mol?L-1 A��E��Һ��pH��A��E�������ƶ�AΪNa2CO3��EΪNaOH��
��1���ɢ��֪���кͼ�������Aֻ������ʹ��ᣬ�����ʵ�����A�ֱ�������ʵ���Ũ��B��C��ϳ����ԣ�C�������٣�˵��B������C���Ա���������B�Ǵ��ᣬ
�ʴ�Ϊ��CH3COOH���кͼ�������Aֻ������ʹ��ᣬ�����ʵ�����A�ֱ�������ʵ���Ũ��B��C��ϳ����ԣ�C�������٣�˵��B������C���Ա���������B�Ǵ��
��2����ˮϡ��0.1mol?L-1 NH3?H2Oʱ���ٽ�NH3?H2O�ĵ��룬��OH-�����ʵ�������NH3?H2O���ʵ���Ũ�ȼ�С���������������OH-Ũ�ȷ�����С����c��H+������
��٢ڼ�С�������¶Ȳ��䣬�۲��䣬������
�ʴ�Ϊ���٢ڣ�
��3����HCl��Һ��εμӵ�Na2CO3��Һ�У����������μӵ�HCl��Na2CO3�����ʵ���ʱ��ǡ������NaHCO3������HCO3-����ˮ��ʼ��ԣ���c��OH-����c��HCO3-����c��Cl-������ˮ����ڵ��룬��c��CO32-����c��OH-��������c��CO32-����c��OH-����c��HCO3-����c��Cl-����
�ʴ�Ϊ��c��CO32-����c��OH-����c��HCO3-����c��Cl-����
��4��pH������2������ʹ�����Һ�зֱ�Ͷ��0.23gNa���ƾ���ȫ��Ӧ��������ȫ��Ӧ������ʣ�࣬���������������ͬ�������Ϊ���ᣬ�����Ũ�ȴ�Ӧ���ʽϴ�HCl��pH�仯�ϴ�����ȷ��ͼ��Ϊ�ڢܣ�
�ʴ�Ϊ���ڢܣ�

��ϰ��ϵ�д�
�����Ŀ
