МвДїДЪИЭ
¶аѕ§№иКЗМ«СфДЬ№в·ьІъТµµДЦШТЄФІДБПЎЈ
(1)УЙКЇУўЙ°їЙЦЖИЎґЦ№иЈ¬ЖдПа№Ш·ґУ¦µДИИ»ЇС§·ЅіМКЅИзПВЈє
SiO2(s)Ј«C(s)=SiO(g)Ј«CO(g)ЎЎ¦¤HЈЅa kJЎ¤molЈ1
2SiO(g)=Si(s)Ј«SiO2(s)ЎЎ¦¤HЈЅb kJЎ¤molЈ1
ўЩ·ґУ¦SiO2(s)Ј«2C(s)=Si(s)Ј«2CO(g)µД¦¤HЈЅ________ kJЎ¤molЈ1(УГє¬aЎўbµДґъКэКЅ±нКѕ)ЎЈ
ўЪSiOКЗ·ґУ¦№эіМЦРµДЦРјдІъОпЎЈёфѕшїХЖшК±Ј¬SiOУлNaOHИЬТє·ґУ¦(ІъОпЦ®Т»КЗ№иЛбДЖ)µД»ЇС§·ЅіМКЅОЄ________________________________ЎЈ
(2)ґЦ№иМбґїіЈјы·Ѕ·ЁЦ®Т»КЗПИЅ«ґЦ№иУлHClЦЖµГSiHCl3Ј¬ѕМбґїєуФЩУГH2»№ФЈє
SiHCl3(g)Ј«H2(g) Si(s)Ј«3HCl(g)ЎЈ
Si(s)Ј«3HCl(g)ЎЈ
І»Н¬ОВ¶Иј°І»Н¬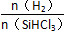 К±Ј¬·ґУ¦ОпXµДЖЅєвЧЄ»ЇВК№ШПµИзНјЛщКѕЎЈ
К±Ј¬·ґУ¦ОпXµДЖЅєвЧЄ»ЇВК№ШПµИзНјЛщКѕЎЈ
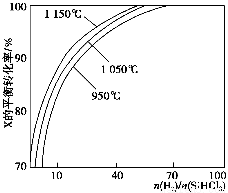
ўЩXКЗ________(МоЎ°H2Ў±»тЎ°SiHCl3Ў±)ЎЈ
ўЪЙПКц·ґУ¦µДЖЅєвіЈКэK(1 150 Ўж)________K(950 Ўж)(МоЎ°>Ў±ЎўЎ°<Ў±»тЎ°ЈЅЎ±)ЎЈ
(3)SiH4(№иНй)·ЁЙъІъёЯґї¶аѕ§№иКЗ·ЗіЈУЕТмµД·Ѕ·ЁЎЈ
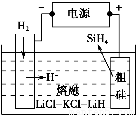
ўЩУГґЦ№иЧчФБПЈ¬ИЫИЪСОµзЅв·ЁЦЖИЎ№иНйФАнИзНјЛщКѕЈ¬µзЅвК±Сфј«µДµзј«·ґУ¦КЅОЄ_________________________________________ЎЈ
ўЪ№и»щМ«СфµзіШРиУГNЎўSiБЅЦЦФЄЛШЧйіЙµД»ЇєПОпYЧч¶Ы»ЇІДБПЈ¬ЛьїЙУЙSiH4УлNH3»мєПЖшМеЅшРРЖшПаіБ»эµГµЅЈ¬ТСЦЄYЦРSiµДЦКБї·ЦКэОЄ60%Ј¬YµД»ЇС§КЅОЄ________ЎЈ
ЎЎ(1)ўЩ2aЈ«bЎЎўЪSiOЈ«2NaOH=Na2SiO3Ј«H2Ўь
(2)ўЩSiHCl3ЎЎўЪ>
(3)ўЩSiЈ«4HЈ«Ј4eЈ=SiH4ЎьЎЎўЪSi3N4
ЎѕЅвОцЎїЎЎ(1)ўЩ·ґУ¦SiO2(s)Ј«2C(s)=Si(s)Ј«2CO(g)їЙУЙµЪТ»ёц·ґУ¦ЎБ2Ј«µЪ¶юёц·ґУ¦µГµЅЈ¬Фт¦¤HЈЅ(2aЈ«b)kJЎ¤molЈ1ЎЈўЪёщѕЭІъОпУР№иЛбДЖј°ЦКБїКШєгµГµЅ·ґУ¦µД»ЇС§·ЅіМКЅОЄSiOЈ«2NaOH=Na2SiO3Ј«H2ЎьЎЈ(2)ўЩёщѕЭНјПсЈ¬ФЪОВ¶ИІ»±дµДЗйїцПВЈ¬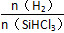 ФцґуЈ¬XµДЖЅєвЧЄ»ЇВКФцґуЈ¬ФтXКЗSiHCl3ЎЈўЪµ±
ФцґуЈ¬XµДЖЅєвЧЄ»ЇВКФцґуЈ¬ФтXКЗSiHCl3ЎЈўЪµ±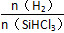 Т»¶ЁК±Ј¬ЙэёЯОВ¶ИЈ¬SiHCl3µДЖЅєвЧЄ»ЇВКФцґуЈ¬Фт±нГч»ЇС§ЖЅєвіЈКэK(1 150 Ўж)>K(950 Ўж)ЎЈ(3)ўЩµзЅвК±Сфј«·ўЙъСх»Ї·ґУ¦Ј¬ґЦ№иК§ИҐµзЧУЈ¬ЅбєПHЈЙъіЙSiH4ЖшМеЈ¬Фтµзј«·ґУ¦КЅОЄSiЈ«4HЈЈ4eЈ=SiH4ЎьЎЈўЪSiЎўNФЄЛШЧйіЙµД»ЇєПОпЦРSiµДЦКБї·ЦКэОЄ60%Ј¬Фт
Т»¶ЁК±Ј¬ЙэёЯОВ¶ИЈ¬SiHCl3µДЖЅєвЧЄ»ЇВКФцґуЈ¬Фт±нГч»ЇС§ЖЅєвіЈКэK(1 150 Ўж)>K(950 Ўж)ЎЈ(3)ўЩµзЅвК±Сфј«·ўЙъСх»Ї·ґУ¦Ј¬ґЦ№иК§ИҐµзЧУЈ¬ЅбєПHЈЙъіЙSiH4ЖшМеЈ¬Фтµзј«·ґУ¦КЅОЄSiЈ«4HЈЈ4eЈ=SiH4ЎьЎЈўЪSiЎўNФЄЛШЧйіЙµД»ЇєПОпЦРSiµДЦКБї·ЦКэОЄ60%Ј¬Фт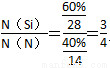 Ј¬јґ»ЇС§КЅОЄSi3N4ЎЈ
Ј¬јґ»ЇС§КЅОЄSi3N4ЎЈ

 »ЖёФМмМмБ·їЪЛгМвїЁПµБРґр°ё
»ЖёФМмМмБ·їЪЛгМвїЁПµБРґр°ёАыУГ»ЇС§ФАнїЙТФ¶Ф№¤і§ЕЕ·ЕµД·ПЛ®Ўў·ПФьµИЅшРРУРР§јмІвУлєПАнґ¦АнЎЈДі№¤і§¶ФЦƸ﹤ҵОЫДаЦРCr(ўу)µДґ¦Ан№¤ТХБчіМИзПВЎЈ

ЖдЦРБтЛбЅюИЎТєЦРµДЅрКфАлЧУЦчТЄКЗCr3Ј«Ј¬ЖдґОКЗFe3Ј«ЎўAl3Ј«ЎўCa2Ј«єНMg2Ј«ЎЈ
(1)КµСйКТУГ18.4 molЎ¤LЈ1µДЕЁБтЛбЕдЦЖ250 mL 4.8 molЎ¤LЈ1µДH2SO4ИЬТєЈ¬ЛщУГµДІЈБ§ТЗЖчіэЙХ±ЎўІЈБ§°фєНБїНІНвЈ¬»№Ри_______________________ _ЎЈ
(2)ЛбЅюК±Ј¬ОЄБЛМбёЯЅюИЎВКїЙІЙИЎµДґлК©УР_____________________________
______________________________________________________(ґріцБЅµг)ЎЈ
(3)H2O2µДЧчУГКЗЅ«ВЛТєўсЦРµДCr3Ј«ЧЄ»ЇОЄCr2O72ЎЄЈ¬РґіцґЛ·ґУ¦µДАлЧУ·ЅіМКЅЈє_____________________________________________________________ЎЈ
(4)іЈОВПВЈ¬Ії·ЦСфАлЧУТФЗвСх»ЇОпРОКЅіБµнК±ИЬТєµДpHИзПВЈє
СфАлЧУ | Fe3Ј« | Mg2Ј« | Al3Ј« | Cr3Ј« |
їЄКјіБµнК±µДpH | 2.7 | Ј | Ј | Ј |
іБµнНкИ«К±µДpH | 3.7 | 11.1 | 8 | 9(>9ИЬЅв) |
јУИлNaOHИЬТєК№ИЬТєіКјоРФЈ¬Cr2O72ЎЄЧЄ»ЇОЄCrO42ЎЄЎЈВЛТєўтЦРСфАлЧУЦчТЄУР________Ј»µ«ИЬТєµДpHІ»ДЬі¬№э8Ј¬ЖдАнУЙКЗ_________________________ЎЈ
(5)ДЖАлЧУЅ»»»КчЦ¬µД·ґУ¦ФАнОЄMnЈ«Ј«nNaRЁDЎъMRnЈ«nNaЈ«Ј¬АыУГДЖАлЧУЅ»»»КчЦ¬іэИҐµДВЛТєўтЦРµДЅрКфСфАлЧУКЗ_______________________________ЎЈ
(6)РґіцЙПКцБчіМЦРУГSO2ЅшРР»№ФК±·ўЙъ·ґУ¦µД»ЇС§·ЅіМКЅЈє__________________________________________________ЎЈ
¶МЦЬЖЪЅрКфФЄЛШјЧЎ«ОмФЪФЄЛШЦЬЖЪ±нЦРµДПа¶ФО»ЦГИзНјЛщКѕЎЈПВБРЕР¶ПХэИ·µДКЗ(ЎЎЎЎ)ЎЈ
јЧ | ТТ |
|
±ы | ¶Ў | Ом |
AЈ®ФЧУ°лѕ¶Јє±ы<¶Ў<Ом BЈ®ЅрКфРФЈєјЧ>±ы
CЈ®ЗвСх»ЇОпјоРФЈє±ы>¶Ў>Ом DЈ®ЧоНвІгµзЧУКэЈєјЧ>ТТ
