��Ŀ����
����������������Ӧ�ù㷺��
��1����ҵ�Ͽ���MnSO4��Һ�������������Mn2O3��Mn2O3�㷺Ӧ���ڵ��ӹ�ҵ��ӡȾ��ҵ��������д���û�ѧ��Ӧ�Ļ�ѧ����ʽ
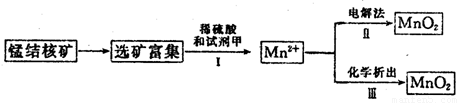
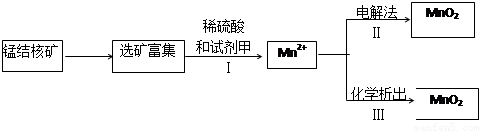
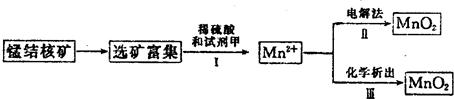
��2�������̲��ŷḻ���̽�˿�����Ҫ�ɷ���MnO2��1991����Allen�����о�����������ϴ��ʹ�ò�ͬ�ķ������Ʊ�������MnO2�����Ʊ�������ͼ��ʾ��
�ٲ���I�У��Լ��ױ�����е�������
a��������b����ԭ��c������
�ڲ�����У���NaClO3Ϊ��������������0.050mol MnO2ʱ������0.10mol?L-1 ��NaClO3��Һ200mL���÷�Ӧ�����ӷ���ʽΪ
��1����ҵ�Ͽ���MnSO4��Һ�������������Mn2O3��Mn2O3�㷺Ӧ���ڵ��ӹ�ҵ��ӡȾ��ҵ��������д���û�ѧ��Ӧ�Ļ�ѧ����ʽ
2MnSO4+Cl2+3H2O�TMn2O3+2H2SO4+2HCl
2MnSO4+Cl2+3H2O�TMn2O3+2H2SO4+2HCl
����2�������̲��ŷḻ���̽�˿�����Ҫ�ɷ���MnO2��1991����Allen�����о�����������ϴ��ʹ�ò�ͬ�ķ������Ʊ�������MnO2�����Ʊ�������ͼ��ʾ��

�ٲ���I�У��Լ��ױ�����е�������
b
b
������ţ���a��������b����ԭ��c������
�ڲ�����У���NaClO3Ϊ��������������0.050mol MnO2ʱ������0.10mol?L-1 ��NaClO3��Һ200mL���÷�Ӧ�����ӷ���ʽΪ
2ClO3-+5Mn2++4H2O=5MnO2+Cl2��+8H+
2ClO3-+5Mn2++4H2O=5MnO2+Cl2��+8H+
����������1��MnSO4��Һ�������������Mn2O3����������������ᣬ�Դ���д��Ӧ����ʽ��
��2���ٲ���I�У�MnԪ�صĻ��ϼ���+4�۽���Ϊ+2�ۣ�
�ڲ�����У���NaClO3Ϊ��������������0.050mol MnO2ʱ������0.10mol?L-1 ��NaClO3��Һ200mL�����õ����غ�ȷ����ԭ����Դ���д���ӷ�Ӧ��
��2���ٲ���I�У�MnԪ�صĻ��ϼ���+4�۽���Ϊ+2�ۣ�
�ڲ�����У���NaClO3Ϊ��������������0.050mol MnO2ʱ������0.10mol?L-1 ��NaClO3��Һ200mL�����õ����غ�ȷ����ԭ����Դ���д���ӷ�Ӧ��
����⣺��1��MnSO4��Һ�������������Mn2O3����������������ᣬ�÷�ӦΪ2MnSO4+Cl2+3H2O�TMn2O3+2H2SO4+2HCl��
�ʴ�Ϊ��2MnSO4+Cl2+3H2O�TMn2O3+2H2SO4+2HCl��
��2���٢ٲ���I�У�MnԪ�صĻ��ϼ���+4�۽���Ϊ+2�ۣ����Լ���Ӧ���л�ԭ�ԣ��ʴ�Ϊ��b��
�ڲ�����У���NaClO3Ϊ��������������0.050mol MnO2ʱ������0.10mol?L-1 ��NaClO3��Һ200mL���軹ԭ������Cl�Ļ��ϼ�Ϊx��
���ɵ����غ��֪��0.05mol����4-2��=0.1mol/L��0.2L����5-x�������x=0�������������������ӷ�ӦΪ2ClO3-+5Mn2++4H2O=5MnO2+Cl2��+8H+��
�ʴ�Ϊ��2ClO3-+5Mn2++4H2O=5MnO2+Cl2��+8H+��
�ʴ�Ϊ��2MnSO4+Cl2+3H2O�TMn2O3+2H2SO4+2HCl��
��2���٢ٲ���I�У�MnԪ�صĻ��ϼ���+4�۽���Ϊ+2�ۣ����Լ���Ӧ���л�ԭ�ԣ��ʴ�Ϊ��b��
�ڲ�����У���NaClO3Ϊ��������������0.050mol MnO2ʱ������0.10mol?L-1 ��NaClO3��Һ200mL���軹ԭ������Cl�Ļ��ϼ�Ϊx��
���ɵ����غ��֪��0.05mol����4-2��=0.1mol/L��0.2L����5-x�������x=0�������������������ӷ�ӦΪ2ClO3-+5Mn2++4H2O=5MnO2+Cl2��+8H+��
�ʴ�Ϊ��2ClO3-+5Mn2++4H2O=5MnO2+Cl2��+8H+��
�������������������仯��������ʿ���������ԭ��Ӧ����ȷ��Ϣ����������MnԪ�صĻ��ϼ۱仯�������غ㼴�ɽ����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ

 MnCl2��Cl2����2H2O
MnCl2��Cl2����2H2O