��Ŀ����
��֪��H2A��A2-�ɱ�ʾS2-��S
��S
��Si
��C
���ӣ�
��1�������£���20mL 0.2mol?L-1 H2A��Һ�еμ�0.2mol?L-1 NaOH��Һ���й������ʵ����仯����ͼ�����Т����H2A�������HA-�������A2-���������ͼʾ��գ�
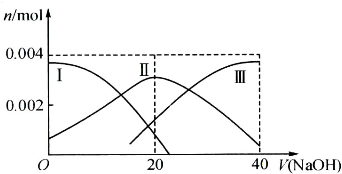
�ٵ�V��NaOH��=20mLʱ����Һ������Ũ�ȴ�С��ϵ�� ��
�ڵ������Ũ�ȵ�NaOH��Һ��H2A��Һ��Ϻ�����Һ��ˮ�ĵ���̶ȱȴ�ˮ �������С������ȡ�������ʹNaHA��Һ�����ԣ����������м��� ��
��2���й�H2A�����ε��Ȼ�ѧ����ʽ���£�
��Na2SO4��s���TNa2S��s��+2O2��g����H1=+1011.0kJ?mol-1��
��2C��s��+O2��g���T2CO��g����H2=-221.0kJ?mol-1��
��Na2SO4��s��+4C��s���TNa2S��s��+4CO��g����H3= kJ?mol-1��
��ҵ���Ʊ�Na2Sʱ����Ҫ���������̼��ͬʱ��Ҫͨ�������Ŀ������������һ������̼��������Ӧ�ų�����ά�ַ�Ӧ�����¶ȣ������ ��
��3����H2AΪ���t��ʱ����pH=2��ϡ�����pH=11��NaOH��Һ�������Ϻ���Һ�����ԣ�����¶���ˮ�����ӻ�����KW= ��
| O | 2- 4 |
| O | 2- 3 |
| O | 2- 3 |
| O | 2- 3 |
��1�������£���20mL 0.2mol?L-1 H2A��Һ�еμ�0.2mol?L-1 NaOH��Һ���й������ʵ����仯����ͼ�����Т����H2A�������HA-�������A2-���������ͼʾ��գ�

�ٵ�V��NaOH��=20mLʱ����Һ������Ũ�ȴ�С��ϵ��
�ڵ������Ũ�ȵ�NaOH��Һ��H2A��Һ��Ϻ�����Һ��ˮ�ĵ���̶ȱȴ�ˮ
��2���й�H2A�����ε��Ȼ�ѧ����ʽ���£�
��Na2SO4��s���TNa2S��s��+2O2��g����H1=+1011.0kJ?mol-1��
��2C��s��+O2��g���T2CO��g����H2=-221.0kJ?mol-1��
��Na2SO4��s��+4C��s���TNa2S��s��+4CO��g����H3=
��ҵ���Ʊ�Na2Sʱ����Ҫ���������̼��ͬʱ��Ҫͨ�������Ŀ������������һ������̼��������Ӧ�ų�����ά�ַ�Ӧ�����¶ȣ������
��3����H2AΪ���t��ʱ����pH=2��ϡ�����pH=11��NaOH��Һ�������Ϻ���Һ�����ԣ�����¶���ˮ�����ӻ�����KW=
��������1���ٸ���ͼ��֪����V��NaOH��=20ʱ��������ӦΪNaOH+H2A�TNaHA+H2O����Һ��ҪΪNaHA������Ϊ������Һ�����ԣ�
�������ӻ���������������ˮ�ģ�����HA-�ĵ���̶Ⱥ�ˮ��̶���Դ�С�жϣ�
��2�����ݸ�˹���ɽ��
��3�����ߵ���������Һ�����ԣ�˵���������������ʵ���Ũ�Ⱥͼ������������ӵ����ʵ���Ũ����ȣ��Ӷ��ó�KW��
�������ӻ���������������ˮ�ģ�����HA-�ĵ���̶Ⱥ�ˮ��̶���Դ�С�жϣ�
��2�����ݸ�˹���ɽ��
��3�����ߵ���������Һ�����ԣ�˵���������������ʵ���Ũ�Ⱥͼ������������ӵ����ʵ���Ũ����ȣ��Ӷ��ó�KW��
����⣺��1���ٵ�V��NaOH��=20 mLʱ��������ӦΪNaOH+H2A�TNaHA+H2O����Һ��ҪΪNaHA��HA-�������ˮ�⣬��Һ�����ԣ���c��Na+����c��HA-����c��H+����c��A2-����c��OH-����
�ʴ�Ϊ��c��Na+����c��HA-����c��H+����c��A2-����c��OH-����
�ڼ�����������������ʱ��HA-�ĵ���̶ȴ���ˮ��̶ȣ���NaHA��Һ�����ԣ������������������ˮ���룬����ʹ����Һ��ˮ�ĵ���̶ȱȴ�ˮ�ĵ���̶�С����ʹ��Һ�����ԣ����Լ����Һ��
�ʴ�Ϊ��С���
��2����Na2SO4��s���TNa2S��s��+2O2��g����H1=+1011.0kJ?mol-1 ��
��2C��s��+O2��g���T2CO��g����H2=-221.0kJ?mol-1 ��
������ʽ��+2���Na2SO4��s��+4C��s���TNa2S��s��+4CO��g����H3=+1 011.0kJ?mol-1+2��-221.0kJ?mol-1��=+569kJ/mol��
�Ʊ�Na2Sʱ����Ҫ���������̼��ͬʱ��Ҫͨ�������Ŀ������������һ������̼��������Ӧ�ų�����ά�ַ�Ӧ�����¶ȣ������ʹ�����Ƶõ���ֻ�ԭ��
�ʴ�Ϊ��+569.0��ʹ�����Ƶõ���ֻ�ԭ��
��3����H2AΪ���t��ʱ����pH=2��ϡ�����pH=11��NaOH��Һ�������Ϻ���Һ�����ԣ�˵���������������ʵ����ͼ������������ӵ����ʵ�����ȣ����������ȣ�����������Ũ�ȵ��ڼ�������������Ũ�ȣ�������������Ũ��Ϊ0.01mol/L��pH=11����Һ������������Ũ��=KW10-11 mol/L��
����0.01mol/L=KW10-11 mol/L��
����KW=10-13��
�ʴ�Ϊ��10-13��
�ʴ�Ϊ��c��Na+����c��HA-����c��H+����c��A2-����c��OH-����
�ڼ�����������������ʱ��HA-�ĵ���̶ȴ���ˮ��̶ȣ���NaHA��Һ�����ԣ������������������ˮ���룬����ʹ����Һ��ˮ�ĵ���̶ȱȴ�ˮ�ĵ���̶�С����ʹ��Һ�����ԣ����Լ����Һ��
�ʴ�Ϊ��С���
��2����Na2SO4��s���TNa2S��s��+2O2��g����H1=+1011.0kJ?mol-1 ��
��2C��s��+O2��g���T2CO��g����H2=-221.0kJ?mol-1 ��
������ʽ��+2���Na2SO4��s��+4C��s���TNa2S��s��+4CO��g����H3=+1 011.0kJ?mol-1+2��-221.0kJ?mol-1��=+569kJ/mol��
�Ʊ�Na2Sʱ����Ҫ���������̼��ͬʱ��Ҫͨ�������Ŀ������������һ������̼��������Ӧ�ų�����ά�ַ�Ӧ�����¶ȣ������ʹ�����Ƶõ���ֻ�ԭ��
�ʴ�Ϊ��+569.0��ʹ�����Ƶõ���ֻ�ԭ��
��3����H2AΪ���t��ʱ����pH=2��ϡ�����pH=11��NaOH��Һ�������Ϻ���Һ�����ԣ�˵���������������ʵ����ͼ������������ӵ����ʵ�����ȣ����������ȣ�����������Ũ�ȵ��ڼ�������������Ũ�ȣ�������������Ũ��Ϊ0.01mol/L��pH=11����Һ������������Ũ��=KW10-11 mol/L��
����0.01mol/L=KW10-11 mol/L��
����KW=10-13��
�ʴ�Ϊ��10-13��
���������⿼����������ʵĵ��롢�������Һ�����жϡ���˹���ɵ�֪ʶ�㣬�ѵ�������Ũ�ȴ�С���жϣ�������Һ�е����ʵ����ˮ��̶���Դ�С��������Ѷ��еȣ�

��ϰ��ϵ�д�
 Сѧ���AB��ϵ�д�
Сѧ���AB��ϵ�д� ABC����ȫ�ž�ϵ�д�
ABC����ȫ�ž�ϵ�д�
�����Ŀ
��16�֣���֪��Ԫ��H2A��ˮ�д������µ��룺H2A===H����HA���� HA��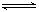 H����A2�����Իش��������⣺
H����A2�����Իش��������⣺
(1)NaHA��Һ��________�ԣ������� ��
(2)ij�¶��£���10 mL 0.1 mol/L NaHA��Һ�м���0.1 mol/L KOH��ҺV mL�����ԣ���ʱ��Һ�����¹�ϵһ����ȷ����________(��д��ĸ)��
| A��c(Na��)��c(K��)=c(HA��)��c(A2��) | B��ˮ�����ӻ�KW��c 2(OH��) 2(OH��) |
| C��V��10 | D��c(K��)��c(Na��) |
 ��֪������CaA��ˮ�д����ܽ�ƽ�⣻CaA(s)
��֪������CaA��ˮ�д����ܽ�ƽ�⣻CaA(s) 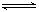 Ca2����A2������H��0��
Ca2����A2������H��0�����¶�����ʱ��Ksp________(���������С�����䡱����ͬ)��
������Һ��ͨ��HCl���壬c(Ca2��)________��ԭ���� ��
(4)�ڴ���CuSO4��5H2O�����г���������Fe2+�����ᴿʱΪ�˳�ȥFe2+�������������������ʹFe2+����ΪFe3+��Ȼ���ټ����ʵ����ʵ�������ҺpH=4��ʹFe3+ת��ΪFe(OH)3����ͬѧ���ɵ�������ҺpH=4�Ƿ��ܴﵽ��ȥFe3+������ʧCu2+��Ŀ�ģ���ͬѧ��Ϊ����ͨ������ȷ�����������й����ϵõ��������ݣ�������Fe(OH)3���ܶȻ�Ksp=8.0��10-38��Cu(OH)2���ܶȻ�Ksp=3.0��10-20��ͨ����Ϊ��������Һ�е�����Ũ��С��1��10-5 mol��L-1ʱ����Ϊ������ȫ������Һ��CuSO4��Ũ��Ϊ3.0 mol��L-1����Cu(OH)2��ʼ����ʱ��Һ��pHΪ________��Fe3+��ȫ����ʱ��Һ��pHΪ_______��ͨ������ȷ����������______������С������С�����

 2PbSO4��s����2H2O��1�������һ��ʱ�����c����d�������ֱ�μӷ�̪�Լ���c��������Һ��죬����˵����ȷ����____________
2PbSO4��s����2H2O��1�������һ��ʱ�����c����d�������ֱ�μӷ�̪�Լ���c��������Һ��죬����˵����ȷ����____________ ��aq����4e��
��aq����4e�� PbSO4��s����2H2O��1��
PbSO4��s����2H2O��1�� H����A2������ش��������⣺
H����A2������ش��������⣺