��Ŀ����
��1 mol��I2(g)��2 mol��H2����2 L�ܱ������У���һ���¶��·�����Ӧ��
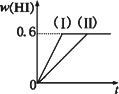
I2(g)��H2(g)![]() 2HI(g)����H��0������ƽ�⣮HI���������w(HI)��ʱ��仯��ͼ����(��)��ʾ��
2HI(g)����H��0������ƽ�⣮HI���������w(HI)��ʱ��仯��ͼ����(��)��ʾ��
(1)��ƽ��ʱ��I2(g)�����ʵ���Ũ��Ϊ________��
(2)���ı䷴Ӧ�������ڼ�������w(HI)�ı仯������(��)��ʾ������������w(HI)�ı仯������(��)��ʾ���������������________����������������________(�����������������)
�ٺ��������£������¶ȣ�
�ں��������£������¶ȣ�
�ۺ��������£���С��Ӧ���������
�ܺ��������£�����Ӧ���������
�ݺ��º��������£������ʵ�������
(3)�������¶Ȳ��䣬����һ����ͬ��2 L�ܱ������м���a mol��I2(g)��b mol��H2(g)��c mol��HI(a��b��c������0)��������Ӧ����ƽ��ʱ��HI�����������Ϊ0.6����a��b��c�Ĺ�ϵ��________��
������
|
����(1)0.05 mol��L��1 ����(2)�ۢݡ��� ����(3)4a��c��2b |

|
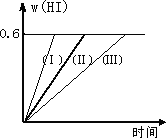
һ���¶��£���1 mol��I2(g)��2 mol��H2����2 L�ܱ������У�������Ӧ��I2(g)��H2(g) �ٺ��������£������¶� �ں��������£���С��Ӧ������� �ۺ��º��������£������ʵ����� �ܺ��º��������£��ټ���1 mol��I2(g)��2 mol��H2 �ݺ��������£�����Ӧ�������
| |
| [����] | |
A�� |
�٢� |
B�� |
�٢ڢ� |
C�� |
�ڢۢ� |
D�� |
�ۢ� |
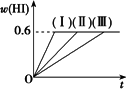
 2HI(g)����H��0�����ﵽƽ�⣮HI���������w(HI)��ʱ��仯������(��)��ʾ���ı�ijһ����������w(HI)�仯������(��)��ʾ���ı�������ǣ�
2HI(g)����H��0�����ﵽƽ�⣮HI���������w(HI)��ʱ��仯������(��)��ʾ���ı�ijһ����������w(HI)�仯������(��)��ʾ���ı�������ǣ�