��Ŀ����
 ij��ѧѧϰС���ͬѧ��̽���ⶨ���ᾧ�壨H2C2O4?xH2O����x��ֵ��ͨ���������ϸ�С��ͬѧ��֪������������ˮ����ˮ��Һ����������KMnO4��Һ������Ӧ2MnO4-+5H2C2O4+6H+�T2Mn2++10CO2��+8H2O������ͬѧ���ø÷�Ӧԭ������˵ζ��ķ����ⶨxֵ��
ij��ѧѧϰС���ͬѧ��̽���ⶨ���ᾧ�壨H2C2O4?xH2O����x��ֵ��ͨ���������ϸ�С��ͬѧ��֪������������ˮ����ˮ��Һ����������KMnO4��Һ������Ӧ2MnO4-+5H2C2O4+6H+�T2Mn2++10CO2��+8H2O������ͬѧ���ø÷�Ӧԭ������˵ζ��ķ����ⶨxֵ���ٳ�ȡ1.260g�����ᾧ�壬�����Ƴ�100.00mLˮ��ҺΪ����Һ��
��ȡ25.00mL����Һ������ƿ�У��ټ���������ϡH2SO4
����Ũ��Ϊ0.1000mol/L��KMnO4����Һ���еζ���ʵ���¼�й��������£�
| �ζ����� | ���������Һ�����mL�� | 0.1000mol/LKMnO4����Һ�����mL�� | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| ��һ�� | 25.00 | 0.00 | 10.02 |
| �ڶ��� | 25.00 | 0.22 | 11.32 |
| ������ | 25.00 | 1.56 | 11.54 |
��1���ζ�ʱ����KMnO4��Һװ����ͼ�е�
��
��
����ס����ҡ����ζ����У���2����ʵ��ζ��ﵽ�յ�ı�־������
���������һ��KMnO4��Һʱ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ������ζ��յ�
���������һ��KMnO4��Һʱ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ������ζ��յ�
����3��ͨ���������ݣ������x=
2
2
����4�������ζ��յ�ʱ���ӵζ��̶ܿȣ����ɴ˲�õ�xֵ��
ƫ��
ƫ��
���ƫ����ƫС�����䡱����ͬ���������ζ�ʱ���õ�KMnO4��Һ����ö�����Ũ�ȱ�С�����ɴ˲�õ�xֵ��
ƫС
ƫС
����������1������KMnO4����ǿ�����ԣ��ḯʴ�ܣ�
��2������KMnO4��Һ��������ɫ��Ϊָʾ���жϵζ��յ�ʱ���ٵμ�KMnO4��Һʱ����Һ������ɫ��Ϊ��ɫ��
��3���������ѧ����ʽ�����ݼ����1.260g�����ᾧ���к�H2C2O4�����ʵ�����Ȼ�����1.260g �����ᾧ���к�H2O�����ʵ���������H2O�����ʵ����ʹ����ᾧ������ʵ����Ĺ�ϵ���x��
���ζ��յ�ʱ���ӵζ��ܶ�������������������KMnO4��Һ�����ƫС���ɴ�����n��H2C2O4��ƫС����n��H2O��ƫ��xƫ��
���ζ�ʱ���õ�KMnO4��Һ����ö�����Ũ�ȱ�С����������������KMnO4��Һ�����ƫ���ɴ�����n��H2C2O4��ƫ����n��H2O��ƫС��xƫС��
��2������KMnO4��Һ��������ɫ��Ϊָʾ���жϵζ��յ�ʱ���ٵμ�KMnO4��Һʱ����Һ������ɫ��Ϊ��ɫ��
��3���������ѧ����ʽ�����ݼ����1.260g�����ᾧ���к�H2C2O4�����ʵ�����Ȼ�����1.260g �����ᾧ���к�H2O�����ʵ���������H2O�����ʵ����ʹ����ᾧ������ʵ����Ĺ�ϵ���x��
���ζ��յ�ʱ���ӵζ��ܶ�������������������KMnO4��Һ�����ƫС���ɴ�����n��H2C2O4��ƫС����n��H2O��ƫ��xƫ��
���ζ�ʱ���õ�KMnO4��Һ����ö�����Ũ�ȱ�С����������������KMnO4��Һ�����ƫ���ɴ�����n��H2C2O4��ƫ����n��H2O��ƫС��xƫС��
����⣺��1����ΪKMnO4����ǿ�����ԣ��ḯʴ�ܣ���Ӧ����ʽ�ζ���ʢװ���ʴ�Ϊ���ף�
��2����KMnO4��Һ��������ɫ��Ϊָʾ���жϵζ��յ�ʱ���ٵμ�KnO4��Һʱ����Һ������ɫ��Ϊ��ɫ��
�ʴ�Ϊ�����������һ��KMnO4��Һʱ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ������ζ��յ㣻
��3����3��2MnO4-+5H2C2O4 +6H+�T2Mn2++10CO2��+8H2O
2 5
0.1000mol/L��0.01L 0.0025mol
25.00mL����Һ�к���0.0025molH2C2O4��100.00mL����Һ�к���0.01molH2C2O4��0.01molH2C2O4������Ϊ0.01mol��90g/mol=0.9g������1.260g�����ᾧ����ˮ�����ʵ���Ϊ1.260g-0.9g=0.36g�������ʵ���Ϊ0.02mol����x=2��
���ζ��յ�ʱ���ӵζ��ܶ�������������������KMnO4��Һ�����ƫС���ɴ�����n��H2C2O4��ƫС����n��H2O��ƫ��xƫ��
ͬ������KMnO4��Һ���ʣ������������ƫ������xֵƫС��
�ʴ�Ϊ��2��ƫ��ƫС��
��2����KMnO4��Һ��������ɫ��Ϊָʾ���жϵζ��յ�ʱ���ٵμ�KnO4��Һʱ����Һ������ɫ��Ϊ��ɫ��
�ʴ�Ϊ�����������һ��KMnO4��Һʱ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ������ζ��յ㣻
��3����3��2MnO4-+5H2C2O4 +6H+�T2Mn2++10CO2��+8H2O
2 5
0.1000mol/L��0.01L 0.0025mol
25.00mL����Һ�к���0.0025molH2C2O4��100.00mL����Һ�к���0.01molH2C2O4��0.01molH2C2O4������Ϊ0.01mol��90g/mol=0.9g������1.260g�����ᾧ����ˮ�����ʵ���Ϊ1.260g-0.9g=0.36g�������ʵ���Ϊ0.02mol����x=2��
���ζ��յ�ʱ���ӵζ��ܶ�������������������KMnO4��Һ�����ƫС���ɴ�����n��H2C2O4��ƫС����n��H2O��ƫ��xƫ��
ͬ������KMnO4��Һ���ʣ������������ƫ������xֵƫС��
�ʴ�Ϊ��2��ƫ��ƫС��
���������⿼���к͵ζ�ʵ�飬�Ѷ����У�ע�����ղ��Ậ���ļ��㷽�����к͵ζ��е����������ɽ��

��ϰ��ϵ�д�
 �¿α�������������ҵ�������γ�����ϵ�д�
�¿α�������������ҵ�������γ�����ϵ�д� ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
�����Ŀ
 �Ҷ����������ᣬij��ѧѧϰС���ͬѧ��̽���ⶨ���ᾧ�壨H2C2O4?xH2O����xֵ��ͨ���������ϸ�С��ͬѧͨ�������ѯ�ã�����������ˮ��ˮ��Һ����������KMnO4��Һ���еζ���
�Ҷ����������ᣬij��ѧѧϰС���ͬѧ��̽���ⶨ���ᾧ�壨H2C2O4?xH2O����xֵ��ͨ���������ϸ�С��ͬѧͨ�������ѯ�ã�����������ˮ��ˮ��Һ����������KMnO4��Һ���еζ���
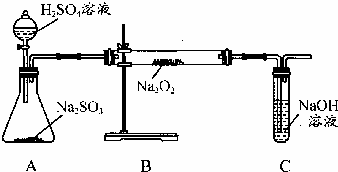 ij��ѧѧϰС���ͬѧΪ̽�������������������ķ�Ӧ������ͼ��ʾ��װ�ý���ʵ�飮ͨ����������������ǵ�ľ�������Թ�C��ľ����ȼ����ش���������
ij��ѧѧϰС���ͬѧΪ̽�������������������ķ�Ӧ������ͼ��ʾ��װ�ý���ʵ�飮ͨ����������������ǵ�ľ�������Թ�C��ľ����ȼ����ش���������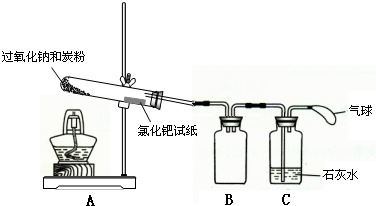 �ƵĻ����ﶼ�й㷺��Ӧ�ã�����Na2O2����һ�ֳ�������������ij��ѧѧϰС���ͬѧ��ͨ������ʵ��̽��̿����Na2O2��Ӧ�IJ��
�ƵĻ����ﶼ�й㷺��Ӧ�ã�����Na2O2����һ�ֳ�������������ij��ѧѧϰС���ͬѧ��ͨ������ʵ��̽��̿����Na2O2��Ӧ�IJ��