��Ŀ����
�α��������Ҵ���������ʵ�顣
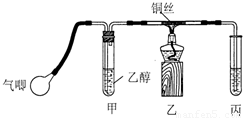
��1����ͬѧ�û�ѧ��������������������ζ����˵��������ij��֣��û�ѧ�����������ӵ��Լ������ֵ���Ҫ�����ǣ� ��������ѧ��֪ʶ�ش�
��2����ͬѧ��̽���������������ζ�����������ʱ��żȻ��������ˮ�м�����������ȩ��Һ�����Կ�����ˮ��ɫ����ͬѧΪ������������������ֲ��룺
��������ȩ����ȡ����Ӧ���� ������ȩ�����л�ԭ�ԣ��彫��ȩ����Ϊ���ᡣ
Ϊ̽�����ֲ�����ȷ����ͬѧ�������������ʵ�鷽����
����һ����pH��ֽ�����ˮ��ɫ����Һ������ԣ�
���������ⶨ��Ӧǰ��ˮ��Br2�����ʵ����ͷ�Ӧ����Һ��Br— ���ӵ����ʵ�����
��3����ͬѧ��Ϊ�������÷�Ӧǰ��ˮ��Br2�����ʵ���Ϊamol������÷�Ӧ��
n(Br—)= mol����˵��������ȩ����ȡ����Ӧ��
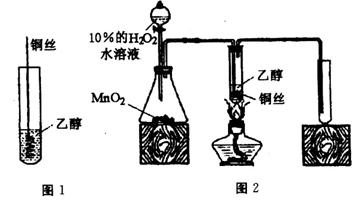
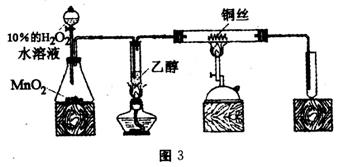
��4����ͬѧ�������ʵ�鷽����
�ٰ����ʵ���֮��Ϊ1:5����KBrO3—KBr��Һ���Ӻ��ʵ��������ᣬ��ȫ��Ӧ��ϡ����1L������0.5molBr2��
��ȡ������Һ10mL����0.005mol Br2������������ȩ��Һ��ʹ֮��ɫ��Ȼ��������Һϡ��Ϊ100mL��ȷ��ȡ����10mL��
�ۼ��������AgNO3��Һ�����ˡ�ϴ�ӡ����������õ�����ɫ����0.188g��������֪CH3COOAg������ˮ����
��ͨ�������жϣ�������ȩ������Ӧ�Ļ�ѧ����ʽΪ
��ͬѧ��Ϊ���ʵ���Ȳ�������Ҳ�������ᣬ����Ϊʲô��
��1��������Һ����������������������ͭ����Һ������ש��ɫ��������2�֣�
��2���� ������ȩ�����ӳɷ�Ӧ ��2�֣�
��3��a ��2�֣�
��4��CH3CHO+Br2+H2O��CH3COOH+2HBr ��2�֣�
Cl- ��SO42-�������Ag+����Ҳ��������������ʵ��Ľ��С� ��2�֣�

 ��У����ϵ�д�
��У����ϵ�д�