��Ŀ����
�α��������Ҵ���������ʵ�飮
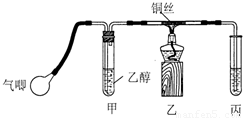
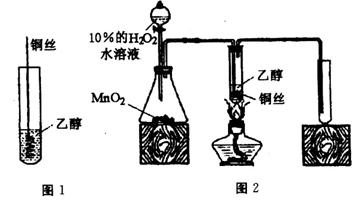
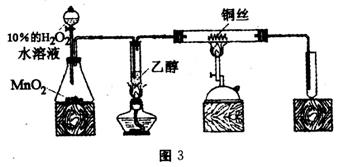
��1����ͬѧ�û�ѧ��������������������ζ����˵��������ij��֣��û�ѧ�����������ӵ��Լ������ֵ���Ҫ�����ǣ� ��������ѧ��֪ʶ�ش�
��2����ͬѧ��̽���������������ζ�����������ʱ��żȻ��������ˮ�м�����������ȩ��Һ�����Կ�����ˮ��ɫ����ͬѧΪ������������������ֲ��룺
��������ȩ����ȡ����Ӧ���� ������ȩ�����л�ԭ�ԣ��彫��ȩ����Ϊ���ᣮ
Ϊ̽�����ֲ�����ȷ����ͬѧ�������������ʵ�鷽����
����һ����pH��ֽ�����ˮ��ɫ����Һ������ԣ�
���������ⶨ��Ӧǰ��ˮ��Br2�����ʵ����ͷ�Ӧ����Һ��Br-���ӵ����ʵ�����
��3����ͬѧ��Ϊ�������÷�Ӧǰ��ˮ��Br2�����ʵ���Ϊamol������÷�Ӧ��
n��Br-��= mol����˵��������ȩ����ȡ����Ӧ��
��4����ͬѧ�������ʵ�鷽����
�ٰ����ʵ���֮��Ϊ1��5����KBrO3-KBr��Һ���Ӻ��ʵ��������ᣬ��ȫ��Ӧ��ϡ����1L������0.5molBr2��
��ȡ������Һ10mL����0.005mol Br2������������ȩ��Һ��ʹ֮��ɫ��Ȼ��������Һϡ��Ϊ100mL��ȷ��ȡ����10mL��
�ۼ��������AgNO3��Һ�����ˡ�ϴ�ӡ����������õ�����ɫ����0.188g��������֪CH3COOAg������ˮ����
��ͨ�������жϣ�������ȩ������Ӧ�Ļ�ѧ����ʽΪ
��ͬѧ��Ϊ���ʵ���Ȳ�������Ҳ�������ᣬ����Ϊʲô�� ��
��1����ͬѧ�û�ѧ��������������������ζ����˵��������ij��֣��û�ѧ�����������ӵ��Լ������ֵ���Ҫ�����ǣ�
��2����ͬѧ��̽���������������ζ�����������ʱ��żȻ��������ˮ�м�����������ȩ��Һ�����Կ�����ˮ��ɫ����ͬѧΪ������������������ֲ��룺
��������ȩ����ȡ����Ӧ����
Ϊ̽�����ֲ�����ȷ����ͬѧ�������������ʵ�鷽����
����һ����pH��ֽ�����ˮ��ɫ����Һ������ԣ�
���������ⶨ��Ӧǰ��ˮ��Br2�����ʵ����ͷ�Ӧ����Һ��Br-���ӵ����ʵ�����
��3����ͬѧ��Ϊ�������÷�Ӧǰ��ˮ��Br2�����ʵ���Ϊamol������÷�Ӧ��
n��Br-��=
��4����ͬѧ�������ʵ�鷽����
�ٰ����ʵ���֮��Ϊ1��5����KBrO3-KBr��Һ���Ӻ��ʵ��������ᣬ��ȫ��Ӧ��ϡ����1L������0.5molBr2��
��ȡ������Һ10mL����0.005mol Br2������������ȩ��Һ��ʹ֮��ɫ��Ȼ��������Һϡ��Ϊ100mL��ȷ��ȡ����10mL��
�ۼ��������AgNO3��Һ�����ˡ�ϴ�ӡ����������õ�����ɫ����0.188g��������֪CH3COOAg������ˮ����
��ͨ�������жϣ�������ȩ������Ӧ�Ļ�ѧ����ʽΪ
��ͬѧ��Ϊ���ʵ���Ȳ�������Ҳ�������ᣬ����Ϊʲô��
��������1��������ȩ�й����ŵ����ʷ�����
��2��������ȩ����ˮ��Ӧ�IJ��������
��3��������ȩ�������ģ���ˮ�е���ȫ�����뷴Ӧ��ȡ����Ӧ������n��Br-��=a mol���ӳɷ�Ӧ��n��Br-��=0 mol��������Ӧ��n��Br-��=2amol��
��4�����ݼ������AgN03��Һ���õ�����ɫ����ΪAgBr������������������ʵ������ٷ�����Ӧ������������е������Ӷ��������ӷ�Ӧ���ɳ�����
��2��������ȩ����ˮ��Ӧ�IJ��������
��3��������ȩ�������ģ���ˮ�е���ȫ�����뷴Ӧ��ȡ����Ӧ������n��Br-��=a mol���ӳɷ�Ӧ��n��Br-��=0 mol��������Ӧ��n��Br-��=2amol��
��4�����ݼ������AgN03��Һ���õ�����ɫ����ΪAgBr������������������ʵ������ٷ�����Ӧ������������е������Ӷ��������ӷ�Ӧ���ɳ�����
����⣺��1��ȩ���ļ��鷽��������������Һ����ȩ���������������жϣ������Ƶ�������ͭ��Һ���Ⱥ�ȩ����Ӧ����ש��ɫ�����������飻
�ʴ�Ϊ��������Һ����������������������ͭ����Һ������ש��ɫ������
��2��������ȩ�������ģ���ˮ�е���ȫ�����뷴Ӧ�����ܷ���ȡ����Ӧ�ӳɷ�Ӧ��������Ӧ��
�ʴ�Ϊ��������ȩ�����ӳɷ�Ӧ��
��3��������ȩ�������ģ���ȩ�й�����Ϊ-CHO����ˮ�е���ȫ�����뷴Ӧ��ȡ����Ӧ������n��Br-��=a mol���ӳɷ�Ӧ��n��Br-��=0 mol��������Ӧ��n��Br-��=2amol��
�ʴ�Ϊ��a��
��4���õ�����0.188gΪAgBr��������n��AgBr��=
=0.001mol��10mL��0.005mol Br2��n��Br-��=2n��Br2������ӦӦΪ������Ӧ�������ӷ���ʽΪ��CH3CHO+Br2+H2O��CH3COOH+2HBr�����ʵ���Ȳ�������Ҳ������������ΪCl-��SO42-�������Ag+����Ҳ��������������ʵ��Ľ��У�
�ʴ�Ϊ��CH3CHO+Br2+H2O��CH3COOH+2HBr��Cl-��SO42-�������Ag+����Ҳ��������������ʵ��Ľ��У�
�ʴ�Ϊ��������Һ����������������������ͭ����Һ������ש��ɫ������
��2��������ȩ�������ģ���ˮ�е���ȫ�����뷴Ӧ�����ܷ���ȡ����Ӧ�ӳɷ�Ӧ��������Ӧ��
�ʴ�Ϊ��������ȩ�����ӳɷ�Ӧ��
��3��������ȩ�������ģ���ȩ�й�����Ϊ-CHO����ˮ�е���ȫ�����뷴Ӧ��ȡ����Ӧ������n��Br-��=a mol���ӳɷ�Ӧ��n��Br-��=0 mol��������Ӧ��n��Br-��=2amol��
�ʴ�Ϊ��a��
��4���õ�����0.188gΪAgBr��������n��AgBr��=
| 0.188g |
| 188g/mol |
�ʴ�Ϊ��CH3CHO+Br2+H2O��CH3COOH+2HBr��Cl-��SO42-�������Ag+����Ҳ��������������ʵ��Ľ��У�
���������⿼����ȩ�������ŵ����ʺͼ���Ӧ�ã�ע��ȡ����Ӧ���ӳɷ�Ӧ��������Ӧ�ķ�Ӧ������������Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ȫ�̽��ϵ�д�
ȫ�̽��ϵ�д�
�����Ŀ