��Ŀ����
ijѧϰС��������кͷ�Ӧԭ����DISϵͳ�������ֻ���Ϣϵͳ���ɴ����������ݲɼ����ͼ������ɣ��ⶨij��ˮ�����ʵ���Ũ�ȣ��Բ�����Һ�����������жϵζ��յ㡣��ش�
��1��ʵ��ʱ��ȡ10.00mL��ˮ��Ʒ�����Ƴ�100mL���ⰱˮ����ò���������Ҫ��������_________________�����������ƣ���
��2����ȡ20.00mL������Һ����ྻ������ƿ�У����Ӻ�DISϵͳ�������ƿ�к�����������ˮ���Ƿ��Ӱ��������_______����ǡ���������ȷ��������
 ��3���ٵζ���ʢ������ǰ��Ҫ��__________________������������ˮϴ�Ӻ���_________________��Ȼ������������Һ������Һ�����͵㴦�ڵζ��ܵ�_________________��
��3���ٵζ���ʢ������ǰ��Ҫ��__________________������������ˮϴ�Ӻ���_________________��Ȼ������������Һ������Һ�����͵㴦�ڵζ��ܵ�_________________��
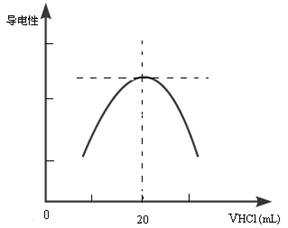
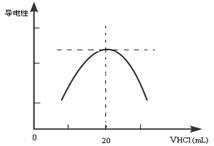
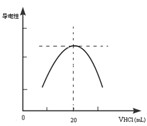
������ƿ�еμ�0.10mol��L��1�����ᣬ�������Ļ����ʾ����Һ����������������������ϵ������ͼ��
д����ˮ�����ᷴӦ�����ӷ���ʽ___________________________���ð�ˮ��Ʒ�����ʵ���Ũ��Ϊ_____________��
��4����һѧϰС������Ϊ������������Դ���һ������Ϊ���ɵ�NH4Cl ��ǿ�������Σ��ᷢ��ˮ���ʹNH4+ Ũ���½���������ǡ����ȫ��ӦʱNH4+ Ũ�Ȳ������ֵ����Һ�����ԾͲ������ֵ������Ϊ��ѧϰС��Ľ����Ƿ���ȷ��________��������___________________��
��1����ʽ�ζ��ܡ��ձ�����������100mL����ƿ����ͷ�ιܣ�5�֣�
��2����1�֣�
��3���ټ��ζ����Ƿ�©ˮ��1�֣�������Ҫʢ�ŵ�������ϴ2��3�Σ�1�֣�����0���̶Ȼ�0���̶����£�1�֣���
��NH3.H2O +H+=NH4++H2O (1��)��1.00mol/L��1�֣�
��4������ȷ��1�֣�����ΪNH4+ˮ�����Һ������Ũ�ȱ��ֲ��䣨1�֣���

 ijѧϰС��������кͷ�Ӧԭ����DISϵͳ�������ֻ���Ϣϵͳ���ɴ����������ݲɼ����ͼ������ɣ��ⶨij��ˮ�����ʵ���Ũ�ȣ��Բ�����Һ�����������жϵζ��յ㣮��ش�
ijѧϰС��������кͷ�Ӧԭ����DISϵͳ�������ֻ���Ϣϵͳ���ɴ����������ݲɼ����ͼ������ɣ��ⶨij��ˮ�����ʵ���Ũ�ȣ��Բ�����Һ�����������жϵζ��յ㣮��ش�