��Ŀ����
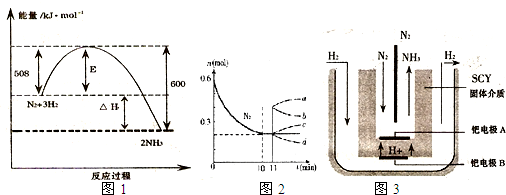
 ��2010?����һģ��ij��ѧѧϰС��Ϊ�о�HA��HB��MOH������Ե����ǿ�����������ʵ������ʵ�飺��pH�T2����������ҺHA��HB��pH=12��MOH����Һ��1mL���ֱ��ˮϡ�͵�1000mL����pH�ı仯����Һ����Ĺ�ϵ��ͼ���������������ݣ���ش��������⣺
��2010?����һģ��ij��ѧѧϰС��Ϊ�о�HA��HB��MOH������Ե����ǿ�����������ʵ������ʵ�飺��pH�T2����������ҺHA��HB��pH=12��MOH����Һ��1mL���ֱ��ˮϡ�͵�1000mL����pH�ı仯����Һ����Ĺ�ϵ��ͼ���������������ݣ���ش��������⣺��1��HAΪ
ǿ
ǿ
�ᣬHBΪ��
��
����ǿ������������2����c=9����ϡ�ͺ��������Һ�У���ˮ�����������Ũ�ȵĴ�С˳��Ϊ
MOH�THA��HB
MOH�THA��HB
�����ᡢ�ѧʽ��ʾ������ϡ�ͺ��HA��Һ��MOH��Һȡ�������ϣ���������Һ��c��A-����c��M+���Ĵ�С��ϵΪ����
����
��������ڡ�����С�ڡ����ڡ�����3����b+c=14����MQHΪ
��
��
��ǿ��������������ϡ�ͺ��HB��Һ��MOH��Һȡ�������ϣ����úܺ���Һ��pH����
����
7������ڡ�����С�ڡ����ڡ�����������1��pH=a��ǿ�ᣬϡ��10n������Һ��pH=a+n��pH=a�����ᣬϡ��10n������Һ��pH����a��a+n֮�䣮�ݴ˿�ȷ����
��2������ˮ�����ӻ�Kw��������Һ��������Ũ�ȣ�����Һ��ˮ����������Ũ�ȵ���ˮ�����������Ũ�ȣ�����Һ��������Ϊˮ���������ӣ����ݼ���Һ��pHֵ����������Ũ�ȣ��ݴ�����
����ͼ��֪HAΪǿ�ᣬMOHΪǿ��ֱ��ˮϡ�͵�1000mL����Һ����������������Ũ����ȣ�
��3�������MOH����Һ��pHֵ���ݴ��жϣ�
��2������ˮ�����ӻ�Kw��������Һ��������Ũ�ȣ�����Һ��ˮ����������Ũ�ȵ���ˮ�����������Ũ�ȣ�����Һ��������Ϊˮ���������ӣ����ݼ���Һ��pHֵ����������Ũ�ȣ��ݴ�����
����ͼ��֪HAΪǿ�ᣬMOHΪǿ��ֱ��ˮϡ�͵�1000mL����Һ����������������Ũ����ȣ�
��3�������MOH����Һ��pHֵ���ݴ��жϣ�
����⣺��1����ͼ��֪��pH�T2����������ҺHA��HB��1mL���ֱ��ˮϡ�͵�1000mL����ҺHA��pH=5������3����λ������HAΪǿ�ᣬ��ҺHB��pHֵ����С��3����λ��HB����Ϊ���ᣮ
�ʴ�Ϊ��ǿ������
��2��pH=5��HA��Һ��c��H+��ˮ=c��OH-��ˮ=
=10-9mol/L������ˮ�����������Ũ��c��H+��ˮ=10-9mol/L��
pH=b��HB��Һ��c��H+��ˮ=c��OH-��ˮ=
=10-��14-b��mol/L��b��5������10-��14-b��mol/L��10-9mol/L��
pH=9��MOH��Һ�У�c��H+��ˮ=1��10-9 mol/L��
����ˮ�����������Ũ��˳��ΪMOH�THA��HB��
pH=12��MOH����Һ1mL����ˮϡ�͵�1000mL��pH=9��pH����3����λ��MOHΪǿ�
��ϡ�ͺ��HA��Һ��MOH��Һȡ�������ϣ�����ǡ����ȫ��Ӧ����ǿ��ǿ���Σ���Һ�����ԣ����ݵ���غ��֪c��A-��=c��M+����
�ʴ�Ϊ��MOH�THA��HB�� ���ڣ�
��3����b+c=14����b=14-c����pH=c��MOH��Һ��c��OH-��=10c-14 mol/L=10-b mol/L��?��c��OH-��=10-2 mol/L��MOHϡ��103����?c��OH-����10-5 mol/L������MOH�������Ϊͬ�¶��£�HB��MOH�ĵ���������ͬ�����Խ�ϡ�ͺ��HB��Һ��MOH��Һȡ�������ϣ���Ӧ����Һ�����ԣ�
�ʴ�Ϊ���������ڣ�
�ʴ�Ϊ��ǿ������
��2��pH=5��HA��Һ��c��H+��ˮ=c��OH-��ˮ=
| 10-14 |
| 10-5 |
pH=b��HB��Һ��c��H+��ˮ=c��OH-��ˮ=
| 10-14 |
| 10-b |
pH=9��MOH��Һ�У�c��H+��ˮ=1��10-9 mol/L��
����ˮ�����������Ũ��˳��ΪMOH�THA��HB��
pH=12��MOH����Һ1mL����ˮϡ�͵�1000mL��pH=9��pH����3����λ��MOHΪǿ�
��ϡ�ͺ��HA��Һ��MOH��Һȡ�������ϣ�����ǡ����ȫ��Ӧ����ǿ��ǿ���Σ���Һ�����ԣ����ݵ���غ��֪c��A-��=c��M+����
�ʴ�Ϊ��MOH�THA��HB�� ���ڣ�
��3����b+c=14����b=14-c����pH=c��MOH��Һ��c��OH-��=10c-14 mol/L=10-b mol/L��?��c��OH-��=10-2 mol/L��MOHϡ��103����?c��OH-����10-5 mol/L������MOH�������Ϊͬ�¶��£�HB��MOH�ĵ���������ͬ�����Խ�ϡ�ͺ��HB��Һ��MOH��Һȡ�������ϣ���Ӧ����Һ�����ԣ�
�ʴ�Ϊ���������ڣ�
���������鶨������ǿ������ʣ��ѶȽϴؼ����pH=a��ǿ�ᣬϡ��10n������Һ��pH=a+n��pH=a�����ᣬϡ��10n������Һ��pH����a��a+n֮�䣨��Ϊ���ͣ�����3��Ϊ�״��㣮

��ϰ��ϵ�д�
�����Ŀ
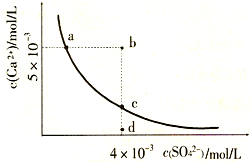 ��2010?����һģ�������£�Ksp��CaS04��=9��l0-6��������CaS04��ˮ�еij����ܽ�ƽ��������ͼ������˵����ȷ���ǣ�������
��2010?����һģ�������£�Ksp��CaS04��=9��l0-6��������CaS04��ˮ�еij����ܽ�ƽ��������ͼ������˵����ȷ���ǣ�������