��Ŀ����
��1������A��B��C��D��E����Ԫ�أ�A��ԭ�Ӻ���û�����ӣ�B��CԪ�ش���ͬһ���ڣ�C��ԭ�Ӱ뾶��С��B��C��������֮��Ϊ27��������֮��Ϊ5��54 g D�ĵ��ʸ��������ᷴӦ������D3����67.2 L����״����������E��C���γ�E2C�����ӻ������E��C��Ԫ�صļ����Ӿ�����ͬ���Ӳ�ṹ��
��B��D������������Ӧˮ�������Ӧ�����ӷ���ʽΪ�� ��
���õ���ʽ��ʾC��E�γ�E2C�Ĺ��̣� ��
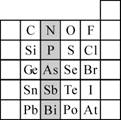
��2��Ԫ�������ڱ��е�λ�ã���ӳ��Ԫ�ص�ԭ�ӽṹ��Ԫ�ص����ʡ���ͼ��Ԫ�����ڱ���һ���֡�
����Ӱ����Ԫ��N��Ԫ�����ڱ��е�λ��Ϊ ��
����һ�������£�S��H2��Ӧ��һ����(������Ϊ��Ӧ���еij̶�)�����жϣ�����ͬ������Se��H2��Ӧ���ȱ�S��H2��Ӧ�� ������ ��(ѡ���������С������ͬ��)
��Br2���н�ǿ�������ԣ�SO2���н�ǿ�Ļ�ԭ�ԣ���SO2����ͨ����ˮ��Ӧ���ӷ���ʽ��__________________________________________��
������˵����ȷ����
A��C��N��O��F��ԭ�Ӱ뾶����ԭ���������������С
B��Si��P��S��ClԪ�صķǽ��������ź˵���������Ӷ���ǿ
C���ɱ�������Һ̬ˮת��Ϊ��̬��Ҫ�˷������ڵĹ��ۼ�
D��HF��HCl��HBr��HI�����ȶ������μ���
��1����Al(OH)3��OH��===AlO2����2H2O
��K����![]()
![]() ����K
����K![]() K��
K��![]() [��
[�� ![]() ]2�� K���������ͷ����2K��Ҳ���֣�
]2�� K���������ͷ����2K��Ҳ���֣�
��2���� �� ��A�ڸ�С �� Br2+SO2+H2O==4H++SO![]() +2Br- �� A BD
+2Br- �� A BD

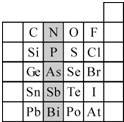
 ��� ��1������A��B��C�������������ģ����ͼ��
��� ��1������A��B��C�������������ģ����ͼ�� ����
����

 ��1������A��B��C��D��E����Ԫ�أ�A��ԭ�Ӻ���û�����ӣ�B��CԪ�ش���ͬһ���ڣ�C��ԭ�Ӱ뾶��С��B��C��������֮��Ϊ27��������֮��Ϊ5��0.96g D�ĵ��ʸ��������ᷴӦ������D3+��1.2L����״����������E��C���γ�E2C�����ӻ������E��C��Ԫ�صļ����Ӿ�����ͬ���Ӳ�ṹ��
��1������A��B��C��D��E����Ԫ�أ�A��ԭ�Ӻ���û�����ӣ�B��CԪ�ش���ͬһ���ڣ�C��ԭ�Ӱ뾶��С��B��C��������֮��Ϊ27��������֮��Ϊ5��0.96g D�ĵ��ʸ��������ᷴӦ������D3+��1.2L����״����������E��C���γ�E2C�����ӻ������E��C��Ԫ�صļ����Ӿ�����ͬ���Ӳ�ṹ��