��Ŀ����
 ��1������A��B��C��D��E����Ԫ�أ�A��ԭ�Ӻ���û�����ӣ�B��CԪ�ش���ͬһ���ڣ�C��ԭ�Ӱ뾶��С��B��C��������֮��Ϊ27��������֮��Ϊ5��0.96g D�ĵ��ʸ��������ᷴӦ������D3+��1.2L����״����������E��C���γ�E2C�����ӻ������E��C��Ԫ�صļ����Ӿ�����ͬ���Ӳ�ṹ��
��1������A��B��C��D��E����Ԫ�أ�A��ԭ�Ӻ���û�����ӣ�B��CԪ�ش���ͬһ���ڣ�C��ԭ�Ӱ뾶��С��B��C��������֮��Ϊ27��������֮��Ϊ5��0.96g D�ĵ��ʸ��������ᷴӦ������D3+��1.2L����״����������E��C���γ�E2C�����ӻ������E��C��Ԫ�صļ����Ӿ�����ͬ���Ӳ�ṹ����д��Ԫ�ط��ţ�A
H
H
��CS
S
��EK
K
����B��D������������Ӧˮ�������Ӧ�����ӷ���ʽΪ��
Al��OH��3+OH-=AlO2-+2H2O
Al��OH��3+OH-=AlO2-+2H2O
�����õ���ʽ��ʾC��E�γ�E2C�Ĺ��̣�
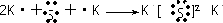
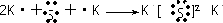
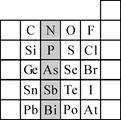
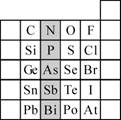
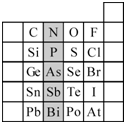
��2��Ԫ�������ڱ��е�λ�ã���ӳ��Ԫ�ص�ԭ�ӽṹ��Ԫ�ص����ʣ���ͼ��Ԫ�����ڱ���һ���֣�
����Ӱ����Ԫ��N��Ԫ�����ڱ��е�λ��Ϊ��
��
��
���ڵ���A
��A
�壮����Ԫ�������ɣ�Ԥ�⣺����ǿ�� H3AsO4
��
��
H3PO4�����á�����������ʾ����Ԫ��S��������ۺ�����۵Ĵ�����Ϊ
+4
+4
����һ�������£�S��H2��Ӧ��һ���ȣ�������Ϊ��Ӧ���еij̶ȣ������жϣ�����ͬ������Se��H2��Ӧ���ȱ�S��H2��Ӧ����С
��С
����ѡ���������С������ͬ������Br2���н�ǿ�������ԣ�SO2���н�ǿ�Ļ�ԭ�ԣ���SO2����ͨ����ˮ����Һ�д��ڵ���Ҫ������
Br-��SO42-��H+
Br-��SO42-��H+
��������˵����ȷ����
A��B��D
A��B��D
A��C��N��O��F��ԭ�Ӱ뾶����ԭ���������������С
B��Si��P��S��ClԪ�صķǽ��������ź˵���������Ӷ���ǿ
C���ɱ�������Һ̬ˮת��Ϊ��̬��Ҫ�˷������ڵĹ��ۼ�
D��HF��HCl��HBr��HI�����ȶ������μ�����
��������1��A��ԭ�Ӻ���û�����ӣ�ӦΪHԪ�أ�B��CԪ�ش���ͬһ���ڣ�C��ԭ�Ӱ뾶��С����C��ԭ����������B��B��C��������֮��Ϊ27��������֮��Ϊ5����B��ԭ������Ϊ11��ΪNaԪ�أ�C��ԭ������Ϊ16��ΪSԪ�أ�
0.96g D�ĵ��ʸ��������ᷴӦ������D3+��1.2L����״��������������Ԫ�صĻ��ϼۺ�ת�Ƶĵ�������֪��
n��H2��=
��n��D��=
��M��D��=
=27��ӦΪAlԪ�أ�
E��C���γ�E2C�����ӻ������E��C��Ԫ�صļ����Ӿ�����ͬ���Ӳ�ṹ����EΪKԪ�أ�
��2������Ԫ���������ڱ��е�λ�ý��Ԫ��������֪ʶ���
0.96g D�ĵ��ʸ��������ᷴӦ������D3+��1.2L����״��������������Ԫ�صĻ��ϼۺ�ת�Ƶĵ�������֪��
n��H2��=
| 1.2L |
| 22.4L/mol |
| ||
| 3 |
| 0.96 | ||||
|
E��C���γ�E2C�����ӻ������E��C��Ԫ�صļ����Ӿ�����ͬ���Ӳ�ṹ����EΪKԪ�أ�
��2������Ԫ���������ڱ��е�λ�ý��Ԫ��������֪ʶ���
����⣺��1��A��ԭ�Ӻ���û�����ӣ�ӦΪHԪ�أ�B��CԪ�ش���ͬһ���ڣ�C��ԭ�Ӱ뾶��С����C��ԭ����������B��B��C��������֮��Ϊ27��������֮��Ϊ5����B��ԭ������Ϊ11��ΪNaԪ�أ�C��ԭ������Ϊ16��ΪSԪ�أ�
0.96g D�ĵ��ʸ��������ᷴӦ������D3+��1.2L����״��������������Ԫ�صĻ��ϼۺ�ת�Ƶĵ�������֪��
n��H2��=
��n��D��=
��M��D��=
=27��ӦΪAlԪ�أ�
E��C���γ�E2C�����ӻ������E��C��Ԫ�صļ����Ӿ�����ͬ���Ӳ�ṹ����EΪKԪ�أ�
�������Ϸ�����֪��AΪHԪ�أ�CΪSԪ�أ�EΪKԪ�أ��ʴ�Ϊ��H��S��K��
��B��D������������Ӧˮ����ֱ�ΪNaOH��Al��OH��3��Al��OH��3�������ԣ�
��Ӧ�����ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O���ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
��C��E�γɵ�E2CΪK2S��Ϊ���ӻ�����õ���ʽ��ʾ���γɹ���Ϊ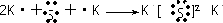 ��
��
�ʴ�Ϊ��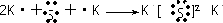 ��
��
��2���ٸ������ڱ���֪��N�����ڱ��еڶ����ڢ�A�壬ͬһ����Ԫ�ش��ϵ���Ԫ�صķǽ��������������Ӧ������������ˮ����������������ʴ�Ϊ��������A������
��Ԫ��S���������Ϊ+6�ۣ������Ϊ-2�ۣ��������Ϊ+4�ۣ�ͬһ����Ԫ�ش��ϵ���Ԫ�صķǽ�����������Խ������������Ӧ����Ӧ����ԽС���ʴ�Ϊ��+4����С��
��Br2���н�ǿ�������ԣ�SO2���н�ǿ�Ļ�ԭ�ԣ���SO2����ͨ����ˮ������ӦΪ��Br2+SO2+2H2O=2Br-+SO42-+4H+��
��Һ�д��ڵ���Ҫ������Br-��SO42-��H+���ʴ�Ϊ��Br-��SO42-��H+��
��A��C��N��O��Fλ��ͬһ���ڣ�������ԭ�Ӱ뾶����ԭ���������������С����A��ȷ��
B��ͬһ���ڴ����ҷǽ���������ǿ����Si��P��S��ClԪ�صķǽ��������ź˵���������Ӷ���ǿ����B��ȷ��
C���ɱ�������Һ̬ˮת��Ϊ��̬��Ҫ�˷����Ӽ�����������C����
D��ͬ����Ԫ�ش��ϵ���Ԫ�صķǽ�������������Ӧ�⻯����ȶ���������HF��HCl��HBr��HI�����ȶ������μ�������D��ȷ��
�ʴ�Ϊ��A��B��D��
0.96g D�ĵ��ʸ��������ᷴӦ������D3+��1.2L����״��������������Ԫ�صĻ��ϼۺ�ת�Ƶĵ�������֪��
n��H2��=
| 1.2L |
| 22.4L/mol |
| ||
| 3 |
| 0.96 | ||||
|
E��C���γ�E2C�����ӻ������E��C��Ԫ�صļ����Ӿ�����ͬ���Ӳ�ṹ����EΪKԪ�أ�
�������Ϸ�����֪��AΪHԪ�أ�CΪSԪ�أ�EΪKԪ�أ��ʴ�Ϊ��H��S��K��
��B��D������������Ӧˮ����ֱ�ΪNaOH��Al��OH��3��Al��OH��3�������ԣ�
��Ӧ�����ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O���ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
��C��E�γɵ�E2CΪK2S��Ϊ���ӻ�����õ���ʽ��ʾ���γɹ���Ϊ
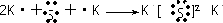 ��
���ʴ�Ϊ��
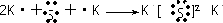 ��
����2���ٸ������ڱ���֪��N�����ڱ��еڶ����ڢ�A�壬ͬһ����Ԫ�ش��ϵ���Ԫ�صķǽ��������������Ӧ������������ˮ����������������ʴ�Ϊ��������A������
��Ԫ��S���������Ϊ+6�ۣ������Ϊ-2�ۣ��������Ϊ+4�ۣ�ͬһ����Ԫ�ش��ϵ���Ԫ�صķǽ�����������Խ������������Ӧ����Ӧ����ԽС���ʴ�Ϊ��+4����С��
��Br2���н�ǿ�������ԣ�SO2���н�ǿ�Ļ�ԭ�ԣ���SO2����ͨ����ˮ������ӦΪ��Br2+SO2+2H2O=2Br-+SO42-+4H+��
��Һ�д��ڵ���Ҫ������Br-��SO42-��H+���ʴ�Ϊ��Br-��SO42-��H+��
��A��C��N��O��Fλ��ͬһ���ڣ�������ԭ�Ӱ뾶����ԭ���������������С����A��ȷ��
B��ͬһ���ڴ����ҷǽ���������ǿ����Si��P��S��ClԪ�صķǽ��������ź˵���������Ӷ���ǿ����B��ȷ��
C���ɱ�������Һ̬ˮת��Ϊ��̬��Ҫ�˷����Ӽ�����������C����
D��ͬ����Ԫ�ش��ϵ���Ԫ�صķǽ�������������Ӧ�⻯����ȶ���������HF��HCl��HBr��HI�����ȶ������μ�������D��ȷ��
�ʴ�Ϊ��A��B��D��
���������⿼��Ԫ�ص�λ�ýṹ���ʵ����ϵ��Ӧ�ã���Ŀ�Ѷ��еȣ�ע�����ԭ�ӵĽṹ�ص�������ڱ��е�λ���ƶ�Ԫ�ص����࣬����Ԫ�������ɵĵݱ���ɣ�

��ϰ��ϵ�д�
�����Ŀ
 ��� ��1������A��B��C�������������ģ����ͼ��
��� ��1������A��B��C�������������ģ����ͼ�� ����
����