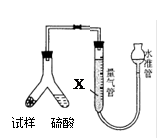
��Ŀ����
ijУ��ѧС���ͬѧ��һ����������·������õ���Cu��Al��Fe������Au��Pt�Ƚ����Ļ���������������Ʊ�����ͭ������������ķ�����

�ش��������⣺
��1���ڢٲ��μӷ�Ӧ�Ľ����� �֡�
��2���ڢڲ�����H2O2����Ϊ��Һ1�к��� ���ӡ�ʹ��H2O2���ŵ��� ��
��3�� �õڢ۲�����CuSO4��5H2O�Ʊ���ˮ����ͭ�ķ����ǣ� ��
��4���������ѧС���ͬѧ���������2��ȡAl2(SO4)3��18H2O ��ʵ�鲽�裺
��ȡ����2������������ ����ַ�Ӧ����ˣ�
��ȡ��Һ������������ ����д�Լ��Ļ�ѧʽ����Ȼ�� ����д����ʵ����������ƣ���
��3��������ϡ�����ܽ⣻
��4����� ����д����ʵ����������ƣ������Al2(SO4)3��18H2O���塣
��1���ڢ۲�����CuSO4��5H2O���п���������Na2SO4��Ϊ�����������ⶨCuSO4��5H2O�Ĵ��ȣ�ѡ��BaCl2(aq)��������Ҫ���Լ������г�����ⶨ�������� ��

�ش��������⣺
��1���ڢٲ��μӷ�Ӧ�Ľ����� �֡�
��2���ڢڲ�����H2O2����Ϊ��Һ1�к��� ���ӡ�ʹ��H2O2���ŵ��� ��
��3�� �õڢ۲�����CuSO4��5H2O�Ʊ���ˮ����ͭ�ķ����ǣ� ��
��4���������ѧС���ͬѧ���������2��ȡAl2(SO4)3��18H2O ��ʵ�鲽�裺
��ȡ����2������������ ����ַ�Ӧ����ˣ�
��ȡ��Һ������������ ����д�Լ��Ļ�ѧʽ����Ȼ�� ����д����ʵ����������ƣ���
��3��������ϡ�����ܽ⣻
��4����� ����д����ʵ����������ƣ������Al2(SO4)3��18H2O���塣
��1���ڢ۲�����CuSO4��5H2O���п���������Na2SO4��Ϊ�����������ⶨCuSO4��5H2O�Ĵ��ȣ�ѡ��BaCl2(aq)��������Ҫ���Լ������г�����ⶨ�������� ��
��1��3 ��1�֣�
��2�� Fe2+ ��1�֣��� ���������ʣ���ԭ������ˮ���Ի�������Ⱦ��1�֣�
��3�������¶ȼ��Ȼ����գ�1�֣�
��4��������������Һ��1�֣� �ڶ�����̼���������ᣬ����ϴ�ӣ�1�֡�2��û��ϴ�ӿ�1�֣���4��������������ȴ�ᾧ������ϴ�� ��1�֡�3��
��5��m����������m�����غ��BaSO4�� ��1�֡�2��������ȷ���֣�
��2�� Fe2+ ��1�֣��� ���������ʣ���ԭ������ˮ���Ի�������Ⱦ��1�֣�
��3�������¶ȼ��Ȼ����գ�1�֣�
��4��������������Һ��1�֣� �ڶ�����̼���������ᣬ����ϴ�ӣ�1�֡�2��û��ϴ�ӿ�1�֣���4��������������ȴ�ᾧ������ϴ�� ��1�֡�3��
��5��m����������m�����غ��BaSO4�� ��1�֡�2��������ȷ���֣�
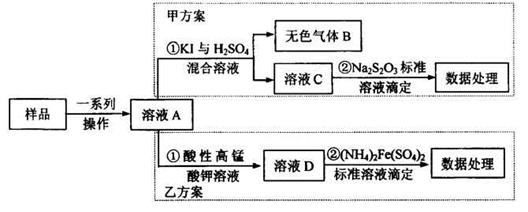
���������39��ϡ���ᡢŨ�����������ȣ�Cu��Al��Fe������Ӧ����Cu2+��Al3+��Fe2+����������1�ijɷ���Pt��Au����Һ1�е�������Cu2+��Al3+��Fe2+���ڢٲ�Cu���ᷴӦ�����ӷ���ʽΪ��Cu+4H++2NO3-
 Cu2++2NO2��+2H2O ��3Cu+8H++2NO3-
Cu2++2NO2��+2H2O ��3Cu+8H++2NO3- 3Cu2++2NO��+4H2O��Au��Pt�� 40���ڢڲ���H2O2�������ǽ�Fe2+����ΪFe3+���������������������������ʣ��Ի�������Ⱦ��41����
3Cu2++2NO��+4H2O��Au��Pt�� 40���ڢڲ���H2O2�������ǽ�Fe2+����ΪFe3+���������������������������ʣ��Ի�������Ⱦ��41���� �۲�����ˮ����ͭ�Ʊ�����ͭ�ķ���Ӧ���������м�����ˮ��42����1���������м�NaOH��Al��OH��3��Ӧ����NaAlO2��������Һ�м�H2SO4����Al2��SO4��3����������ȴ���ᾧ�����˿ɵ����������壻����ԭ�����ýǶȿ��Ƿ����Ҹ���������Ϊ���ӵ�NaOH���Ʊ���Al2��SO4��3��ԭ�����û�й�ϵ�����ԭ���˷ѣ���2��������̼���������ᣬ����ϴ�ӣ���4�����������������ȴ�ᾧ������ϴ�� �����Al2(SO4)3��18H2O���塣 43���������m����������m�����غ��BaSO4����������

��ϰ��ϵ�д�
�����Ŀ
 N2O4��H��0
N2O4��H��0