��Ŀ����
���л�ѧ���̼��������ȷ���� �� ��
A����NaHSO4��Һ�е���Ba(OH)2��Һ������H+��SO42-��Ba2+��OH-��BaSO4����H2O
B����ˮ�����c(H+)Ϊ10-3 mol��L-1����Һ�У�Na+��NO3-��SO32-��Cl-һ���ܴ�������
C������1molKAl(SO4)2����Һ�м���Ba(OH)2��Һ�������������ʱ���������ܵ����ʵ���Ϊ2mol
D��������Ũ�����ữ��KMnO4��Һ��H2O2��ϣ���֤��H2O2���л�ԭ��2MnO4-��6H+��5H2O2��2Mn2+��5O2����8H2O
C
��������
���������
���⿼��������������������ӹ��漰�����йص����ӷ���ʽ��д��A.Ҫʹ����Һ�����ԣ���ô
H+��OH-��������1:1��1molNaHSO4�����1molH+��1molBa(OH)2�����2molOH-������n(NaHSO4):n[Ba(OH)2]=2:1���������ӷ���ʽӦΪ��2H+��SO42-��Ba2+��2OH-��BaSO4����2H2O��B����ˮ�����c(H+)Ϊ10-3 mol��L-1��˵������Һ�Ǵٽ���ˮ�ĵ��롣ˮ��c(H+)=10-7mol��L-1�����Ը���Һ�бغ������������ӣ�Fe3+�� Cu2+ ��Al3+�ȣ�����������ӣ�CO32-��CH3COO-�ȣ��������Һ�д���Fe3+��Cu2+�Ļ�����ô��Ȼ��SO32-���ܴ������档D��KMnO4��Һ������Ũ�����ữ������������KMnO4��Һ����Ũ���ᷢ����Ӧ��Ҳ������ŨHNO3�ữ����ŨHNO3��������ǿ�����ԣ���C��Al3++2SO42-+ 2Ba2+��4OH-= 2BaSO4��+ AlO2-+2H2O�����������ʵ���Ϊ2mol������Ϊ466g��Al3++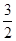 SO42-+
SO42-+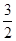 Ba2+��3OH-=
Ba2+��3OH-=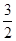 BaSO4��+ Al(OH)3������������£����������ʵ���Ϊ2.5mol��������Ϊ427.5g������C��ȷ��
BaSO4��+ Al(OH)3������������£����������ʵ���Ϊ2.5mol��������Ϊ427.5g������C��ȷ��
���㣺�����������������йأ������ӷ���ʽ����д
