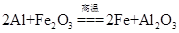��Ŀ����
��һ��ɫ����Һ�����ܺ�Al3����Fe3����Mg2����Na����CO32-��Cl����NO3-�������е������֡���������ʵ�飺
һ��ȡ��������Һ�������������ữ��AgNO 3��Һ���а�ɫ�������ɡ�
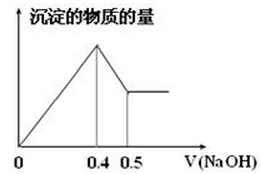
������ȡ������Һ�������������ƣ��а�ɫ���������������������Ƶ��������ɰ�ɫ��������������ͼ��ʾ��
���ƶϣ�
��1������Һ��һ������__________ ____��һ��������________________��
��2����ͼ��֪��ɫ��������__________�֣��ֱ���__________________���ѧʽ���������ʵ�����Ϊ ��
��3�� д��ͼ��a b�仯���̵����ӷ���ʽ ��
b�仯���̵����ӷ���ʽ ��
һ��ȡ��������Һ�������������ữ��AgNO 3��Һ���а�ɫ�������ɡ�
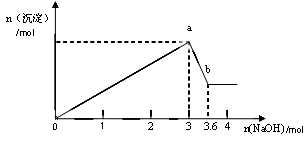
������ȡ������Һ�������������ƣ��а�ɫ���������������������Ƶ��������ɰ�ɫ��������������ͼ��ʾ��

���ƶϣ�
��1������Һ��һ������__________ ____��һ��������________________��
��2����ͼ��֪��ɫ��������__________�֣��ֱ���__________________���ѧʽ���������ʵ�����Ϊ ��
��3�� д��ͼ��a
 b�仯���̵����ӷ���ʽ ��
b�仯���̵����ӷ���ʽ ����1��Al3����Mg2����Cl�� �� Fe3����CO32-
��2��2�� Al(OH)3��Mg(OH)2 �� 1:1
��3��Al(OH)3+ OH�� AlO2��+ 2H2O
AlO2��+ 2H2O
��2��2�� Al(OH)3��Mg(OH)2 �� 1:1
��3��Al(OH)3+ OH��
 AlO2��+ 2H2O
AlO2��+ 2H2O ���������������Һ����ɫ���ģ����ԣ������ų�Fe3���Ĵ��ڣ���ΪFe3������Һ���ػ�ɫ��ȡ��������Һ�������������ữ��AgNO 3��Һ���а�ɫ�������ɣ�˵������Һ��һ������Cl������Ϊֻ��AgCl��ɫ��Һ�Dz�����ϡHNO3�ġ���ȡ������Һ����������������Һ������Ϊ���г������������ʧ��˵��ԭ��Һ��һ������Al3����Mg2����ͬʱ�ų���CO32-�Ĵ��ڣ���ΪAl3����Mg2����CO32--�ᷴӦ�����ܴ������档��Na����NO3-�治���ڶ�������������Ӱ�졣�����������ﵽ���ʱ���ټ�������������Һ������������ʧ����ԭ�������ɵ����������������������������������������Һ������Ӧ����ƫ�����ƺ�ˮ����������þ��������������������Һ��Ӧ��

��ϰ��ϵ�д�
 ����������ϵ�д�
����������ϵ�д�
�����Ŀ

 CuY2-+2H+��д������CuSO4��5H2O���������ı���ʽw=�� ;
CuY2-+2H+��д������CuSO4��5H2O���������ı���ʽw=�� ;