��Ŀ����
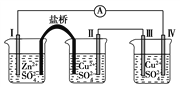
����Ŀ�����Ȼ���������[Co(NH3)6]Cl3 �dzȻ�ɫ������ˮ�������Ǻϳ�����һЩ����������ԭ�ϡ���ͼ��ij����С���Ժ��ܷ��ϣ�������Fe��Al �����ʣ���ȡ[Co(NH3)6]Cl3 �Ĺ������̣�

�ش��������⣺
��1��д���ӡ�����NaClO3��������Ӧ�����ӷ���ʽ______________��
��2������Na2CO3 ��pH��a�����������ֳ������ֱ�Ϊ_______________________���ѧʽ����
��3��������IJ������_____________________________����ȴ�ᾧ����ѹ���ˡ�
��4��������NH4Cl������Ӧ���⣬���ɷ�ֹ�Ӱ�ˮʱc(OH��) ������ԭ����_________________��
��5�������������裬��ͬѧ��ΪӦ�ȼ��백ˮ�ټ���H2O2����ͬѧ��Ϊ�Լ�����˳��Բ�����Ӱ�졣����Ϊ___________����ס����ҡ���ͬѧ�۵���ȷ��������_________________________________��д���ò���Ļ�ѧ����ʽ��________________________________
��6��ͨ���������ɲⶨ��Ʒ���ܵĺ������� [Co(NH3)6]Cl3 ת����Co3���������KI ��Һ������Na2S2O3��Һ�ζ�(������Һ��ָʾ��)����Ӧԭ����2Co3����2I����2Co2����I2��I2��2S2O32����2I����S4O62����ʵ������У����в����ᵼ�������ܺ�����ֵƫ�ߵ���_______��
a���þ����ڿ����е� KI ����������Һ
b��ʢװNa2S2O3��Һ�ļ�ʽ�ζ���δ��ϴ
c���ζ��������ֵζ�����������
d����Һ��ɫ��ȥ����������
���𰸡�6Fe2����ClO3����6H��=6Fe3����Cl����3H2O Fe(OH)3 �� Al(OH)3 HCl��Χ������Ũ�� NH4Cl����ˮ�����NH4�������ƺ��ڼ����NH3��H2O�ĵ��� �� ��ֹCo(OH)3������ H2O2��2CoCl2��2NH4Cl��10NH3��H2O��2Co(NH3)6Cl3����12H2O ab
��������
�Ժ��ܷ��ϣ�������Fe��Al �����ʣ���ȡ[Co(NH3)6]Cl3 ���������ܽ���ϣ��õ�Co2+��Fe2+��Al3+��������Һ������������NaClO3��������������Ϊ�����ӣ��ټ���̼���Ƶ���pH�����������ӡ�������Ϊ�����������������������ˣ��õ���Һ������Co2+����Һ�м����������pH=2-3,�������̿���Ȼ����Һ�õ�CoCl2 6H2O�������μ��백ˮ�������⣬������ӦH2O2��2CoCl2��2NH4Cl��10NH3��H2O��2Co(NH3)6Cl3����12H2O���ٽ��������Ȼ����Ϊ������Ũ������ȴ�ᾧ����ѹ���˵õ���Ʒ���ݴ˷�������
(1)������NaClO3��Ŀ���ǽ�������������Ϊ�����ӣ����������ӷ�ӦΪ6Fe2����ClO3����6H��=6Fe3����Cl����3H2O��
(2)����̼���Ƶ���pH�����������Ӻ������ӣ�ת��ΪFe(OH)3 �� Al(OH)3��
(3)����ֹ��Ʒˮ�⣬��Ӧ��HCl��Χ������Ũ����
(4)�������Ȼ�麟���Ϊ��Ӧ���⣬NH4Cl����ˮ�����NH4�������ƺ��ڼ����NH3��H2O�ĵ��룬�ɷ�ֹ�Ӱ�ˮʱ����������Ũ�ȹ���
(5)���ȼ���������⣬����Ԫ��������Co3+������백ˮ���������������ܣ������ڲ�Ʒ�����ɣ��ʼ�ͬѧ��ȷ���ȼ��백ˮ�ټ���������⣬�ɷ�ֹCo(OH)3�����ɣ���ʱ�ķ�ӦΪH2O2��2CoCl2��2NH4Cl��10NH3��H2O��2Co(NH3)6Cl3����12H2O��
(6) a���þ����ڿ����е� KI ����������Һ���⻯�ز��ֱ�����Ϊ�ⵥ�ʣ��ζ�ʱ���ĵ���������Ʊ����࣬�ⶨ���ƫ�ߣ�����ȷ��
b��ʢװNa2S2O3��Һ�ļ�ʽ�ζ���δ��ϴ�����Ҳ��ϡ�ͣ��ζ�ʱ���ĵ���������Ʊ�Ҳ������࣬�ⶨ���ƫ�ߣ�����ȷ��
c���ζ��������ֵζ����������ݣ�����ռ����������ĵ���������Ʊ�Һ�������ƫС���ⶨ���ƫ�ͣ��ʴ���
d����Һ��ɫ��ȥ��������������ʱ��Һ��ϲ������ⵥ�ʲ�δ��ȫ����Һ��Ӧ��ȫ���������ĵı�Һ���٣��ⶨ���ƫ�ͣ��ʴ���ѡab��