��Ŀ����
14����ҵ���Ʊ�BaCl2�Ĺ�������ͼ��ͼ��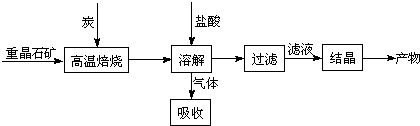
ij�о�С����ʵ�������ؾ�ʯ����Ҫ�ɷ�BaSO4���Թ�ҵ���̽���ģ��ʵ�飮�����
BaSO4��s��+4C��s��$\stackrel{����}{?}$4CO��g��+BaS��s����H1=+571.2kJ•mol-1 ��
BaSO4��s��+2C��s��$\stackrel{����}{?}$2CO2��g��+BaS��s����H2=+226.2kJ•mol-1 ��
��1�������ù���NaOH��Һ���գ��õ����ƣ�Na2Sˮ������ӷ���ʽΪS2-+H2O?HS-+OH-��HS-+H2O?H2S+OH-��
��2����BaCl2��Һ�м���AgNO3��KBr�������ֳ�������ʱ��$\frac{c��B{r}^{-}��}{c��C{l}^{-}��}$=2.7��10-3��
[Ksp��AgBr��=5.4��10-13��Ksp��AgCl��=2.0��10-10]
��3����ӦC��s��+CO2��g��$\stackrel{����}{?}$2CO��g���ġ�H=+172.5kJ•mol-1��
���� ��1��Na2Sˮ��ʼ��ԣ���������ˮ�⣬�Ե�һ��ˮ��Ϊ�����ݴ�д����ˮ������ӷ���ʽ��
��2�������ܶȻ���������$\frac{c��B{r}^{-}��}{c��C{l}^{-}��}$��
��3�����ø�˹���ɼ���֪�Ȼ�ѧ����ʽ����÷�Ӧ���ʱ䣮
��� �⣺��1��Na2Sˮ��ʼ��ԣ���������ˮ�⣬�Ե�һ��ˮ��Ϊ������һ��ˮ����������HS-���ڶ���ˮ������H2S��ˮ������ӷ���ʽ�ֱ�ΪS2-+H2O?HS-+OH-��HS-+H2O?H2S+OH-��
�ʴ�Ϊ��S2-+H2O?HS-+OH-��HS-+H2O?H2S+OH-��
��2�������ֳ�������ʱ��Ag+����Ũ����ͬ�������ܶȻ��������㣬c��Br-��=$\frac{{K}_{sp}��AgBr��}{c��A{g}^{+}��}$��c��Cl-��=$\frac{{K}_{sp}��AgCl��}{c��A{g}^{+}��}$����$\frac{c��B{r}^{-}��}{c��C{l}^{-}��}$=$\frac{5.4��1{0}^{-13}}{2.0��1{0}^{-10}}$=2.7��10-3��
�ʴ�Ϊ��2.7��10-3��
��3��BaSO4��s��+4C��s��$\stackrel{����}{?}$4CO��g��+BaS��s����H1=+571.2kJ•mol-1 ��
BaSO4��s��+2C��s��$\stackrel{����}{?}$2CO2��g��+BaS��s����H2=+226.2kJ•mol-1 ��
���ݸ�˹����$\frac{��-��}{2}$�ɵã�
C��s��+CO2��g��$\frac{\underline{\;����\;}}{\;}$2CO��g����H=$\frac{571.2kJ•mo{l}^{-1}-226.2kJ•mo{l}^{-1}}{2}$=+172.5kJ•mol-1��
�ʴ�Ϊ��+172.5��
���� ���⿼���˹�����ڷ�Ӧ���еļ��㡢�������ܶȻ��ļ��㣬��Ŀ�Ѷ��еȣ������Ϊ�ۺϣ��漰�����ˮ�⡢���ܵ���ʵ��ܽ�ƽ�⡢��Ӧ�ȵļ����֪ʶ��ע�����ո�˹���ɵ����ݼ�Ӧ�÷�������ȷ�ܶȻ��ĸ���ε�ˮ��ԭ��������������ѧ�������Ӧ��������

| A�� | p��C2H2����p��C2H4����p��C2H�� | B�� | p��C2H4����p��C2H����p��C2H2�� | ||
| C�� | p��C2H2����p��C2H����p��C2H4�� | D�� | p��C2H����p��C2H4����p��C2H2�� |
| A�� | �¶ȼƵ�ˮ����Ӧ������ƿ������ˮ�� | |
| B�� | �������е�ˮ�������Ǵ��¿ڽ��룬�Ͽ��ų� | |
| C�� | ʵ������Ҫ����ƿ�м��뼸�����Ƭ����ֹ���ֱ������� | |
| D�� | ��ƿ������ʯ�������� |
| A�� | pH=0����Һ������ | |
| B�� | ��ҺpH���3����c��H+�����30�� | |
| C�� | pH=5��HCl��ˮϡ��1000������ҺpH=8 | |
| D�� | pH=5��CH3COOH��Һ���Ա�pH=6��HCl��Һ������ǿ |
| A�� | ������̼̼���� | B�� | �������Ҵ� | C�� | ������� | D�� | ������������ |
| A�� | 1mol/L��KNO3��Һ��H+��Fe2+��Cl-��SO42- | |
| B�� | Na+��OH-��Fe2+��S2- | |
| C�� | ������Һ��Na+��SO42-��AlO2-��SO32- | |
| D�� | ������Һ�У�MnO4-��I-��Na+��Al3+ |
| A�� | 15 g����-CH3�������еĵ�������NA | |
| B�� | 0.5 mol 1��3-����ϩ�����к���C�TC����ΪNA | |
| C�� | ��״���£�1 L������ȼ�պ����ɵ���̬����ķ�����Ϊ$\frac{5}{22.4}$NA | |
| D�� | ���³�ѹ�£�1 mol���������еĹ��ۼ���ĿΪ12NA |
| A�� | CO2��ˮ��Һ��c��H+����c��HCO3-����2c��CO32-�� | |
| B�� | ��Ũ�ȵ�HCN��Һ��Na0H��Һ�������ϣ�������ҺpH��7������Һ������Ũ�ȣ�c��Na+����c��CN-����c��OH-����c��H+�� | |
| C�� | 0.4mol•L-1ijһԪ��HA��Һ��0.2mol•L-1Na0H��Һ�������ϵ���Һ�У�2c��OH-��+c��A-��=2c��H+��+c��HA�� | |
| D�� | ��������HX��HY��Ϻ���Һ�е�c��H+��Ϊ��KaΪ����ƽ�ⳣ������c��H+��=$\frac{{K}_{a}��HX��•c��HX��}{c��{X}^{-}��}$+$\frac{{K}_{a}��HY��•c��HY��}{c��{Y}^{-}��}$+c��OH-�� |