��Ŀ����
����Ŀ����֪25�桢101kPaʱ��һЩ���ʵ�ȼ����Ϊ��
��ѧʽ | CO(g) | H2(g) | CH3OH(l) | CH4(g) |
��H/(kJ/mol) | -283.0 | -285.8 | -726.51 | -890. 31 |
��ش��������⣺
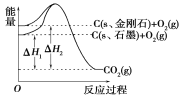
��1�����ݸ�˹����������з�Ӧ���Ȼ�ѧ����ʽ��
CO(g)+2H2(g)==CH3OH(l) ��H=_________��
��2������H2��CH4�Ļ������112 L����״������ʹ����ȫȼ������CO2(g)��H2O(l)�����ų�����3 242.5 kJ����ԭ���������H2��CH4�����ʵ���֮���ǣ�____________��
A.1:1 B.1:3 C.1:4 D.2:3
���𰸡�-128.09 kJ/molD
����������1��COȼ�յ��Ȼ�ѧ����ʽ��CO��g��+1/2O2��g���TCO2��g����H=-283.0 kJ��mol��1 ����H2ȼ�յ��Ȼ�ѧ����ʽ��2H2��g��+O2��g���T2H2O��l����H=-285.8��2 kJ��mol��1 ����CH3OHȼ�յ��Ȼ�ѧ����ʽ��CH3OH��l��+3/2O2��g���TCO2��g��+2H2O��l����H=-726.5 kJ��mol��1 ��������+��+��-�����ɵã�CO��g��+2H2��g���TCH3OH��l����H=-128.1KJ��mol��1
�ʴ�Ϊ��-128.1KJ��mol��1��
(2)H2��CH4�Ļ������112L����״�������ʵ���Ϊ112L/22.4L��mol��1=5mol�����Ի�������ƽ��ȼ����ΪΪ3242.5/5kJ��mol��1=648.5kJ��mol��1����H2��g��+1/2O2��g��=H2O��1����H=-285.8kJ��mol��1��֪��������ȼ����Ϊ285.8kJ��mol��1����CH4��g��+2O2��g��=CO2��g��+2H2O��1����H=-890.3kJ��mol��1��֪�������ȼ����Ϊ890.3kJ��mol��1��
����ʮ�ֽ��淨����ԭ���������H2��CH4�����ʵ���֮�ȣ�

����ԭ���������H2��CH4�����ʵ���֮��Ϊ241.8kJ��mol��1��362.7kJ��mol��1=2��3��
��ѡ��D��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ������þ����(MgSO4��7H2O)��һ����Ҫ�Ļ���ԭ�ϣ��������Ƹըҩ����ֽ�������ȡ�����þ��(��Ҫ�ɷ���̼��þ��������FeCO3�Ͳ���������)Ϊԭ����ȡ����þ������������£�

��֪������������������������ʽ����ʱ��Һ��pH���±���
������ | Mg2�� | Fe2�� | Fe3�� |
��ʼ���� | 9.1 | 7.6 | 1.9 |
��ȫ���� | 11.1 | 9.7 | 3.2 |
(1) �������������У�����H2O2��Һ��Ŀ����________(�����ӷ���ʽ��ʾ)���������������У��ð�ˮ����pH�ķ�Χ��________��
(2) �����ˡ�������Һ�к��е�������ΪMg2����________��
(3) ���ᾧ��������������Ũ����Һ��ʹ�õ�ʵ������������̨(����Ȧ)���ƾ��ơ���������____________________����
(4) �ⶨ����þ������Ʒ��MgSO4��7H2O�ĺ�������������ʵ�鷽����
��. ��ȡ��������þ������Ʒ1.500 g���������EDTA�����100 mL pH��9��10֮�����ҺA��
��. ��ȡ25.00 mL��ҺA����ƿ�У���0.1000 mol��L��1 Zn2������Һ�������EDTA��Ӧ������Zn2������Һ20.00 mL��
��. ����ȡ25.00 mL��ҺA����һֻ��ƿ�У�����pHΪ5��6����0.100 0 mol��L��1 Zn2������Һ��֮��ַ�Ӧ������Zn2������Һ35.00 mL��
��֪����pHΪ9��10ʱ��Mg2����Zn2��������EDTA(H2Y2��)��Ӧ��Mg2����H2Y2��===MgH2Y����Zn2����H2Y2��===ZnH2Y
��pHΪ5��6ʱ��Zn2������EDTA��Ӧ��������MgH2Y��Ӧ��Zn2����MgH2Y===ZnH2Y��Mg2��
����Ʒ�����ʲ����뷴Ӧ��
��������þ������Ʒ��MgSO4��7H2O����������________��(д���������)��