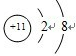��Ŀ����
���е�ʵ�߱�ʾԪ�����ڱ��IJ��ֱ߽磮�١���ֱ��ʾԪ�����ڱ��ж�Ӧλ�õ�Ԫ�أ�
��1��Ԫ�����ڱ�������Ԫ�������ɣ�Ԫ�������ɵı�����
��2��Ԫ�آۢܢݢ�ԭ�Ӱ뾶�ɴ�С��˳���ǣ�
��3������ЩԪ�ص�����������Ӧˮ�����У�������ǿ����
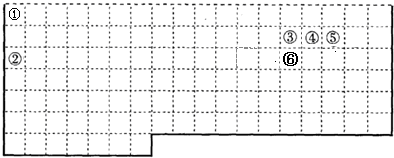
��4����Ҫ����ͼ���ݵ�ԭ�ӽṹʾ��ͼ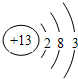
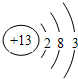
��5��������нϻ��õ���
��6��д����ͼ��Ԫ�ء�E�������ڱ��е�λ��
| ��A | ��A | ||||||||||||||||
| �� | �� | �� | |||||||||||||||
| �� | ��B | ��B | ��B | �� | �� | �� | �� | ||||||||||
| �� | Fe | �� | |||||||||||||||
| E | |||||||||||||||||
Ԫ��ԭ�Ӻ�������Ų��������Ա仯
Ԫ��ԭ�Ӻ�������Ų��������Ա仯
����2��Ԫ�آۢܢݢ�ԭ�Ӱ뾶�ɴ�С��˳���ǣ�
Na��Al��S��F
Na��Al��S��F
����Ԫ�ط��ű�ʾ������3������ЩԪ�ص�����������Ӧˮ�����У�������ǿ����
HClO4
HClO4
����д��ѧʽ����ͬ����������Ϊ������
������
��������ǿ����KOH
KOH
�������Ե���Al��OH��3
Al��OH��3
��4����Ҫ����ͼ���ݵ�ԭ�ӽṹʾ��ͼ
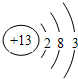
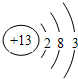
��5��������нϻ��õ���
��
��
����дԪ�����ƣ����û�ѧ����ʽ��ʾԭ��Cl2+2NaBr=2NaCl+Br2
Cl2+2NaBr=2NaCl+Br2
��6��д����ͼ��Ԫ�ء�E�������ڱ��е�λ��
�������ڵ�IVA��
�������ڵ�IVA��
����������Ԫ����Ԫ�����ڱ��е�λ�ÿ�֪����ΪN����ΪO����ΪF����ΪNa����ΪAl����ΪS����ΪCl����ΪAr����ΪK����ΪBr��EΪSn��Ȼ������Ԫ�ؼ��䵥�ʡ�����������������
����⣺��Ԫ����Ԫ�����ڱ��е�λ�ÿ�֪����ΪN����ΪO����ΪF����ΪNa����ΪAl����ΪS����ΪCl����ΪAr����ΪK����ΪBr��EΪSn��
��1��Ԫ�������ɵı���ΪԪ��ԭ�Ӻ�������Ų��������Ա仯����Ԫ�����ʵ������Ա仯���ʴ�Ϊ��Ԫ��ԭ�Ӻ�������Ų��������Ա仯��
��2�����Ӳ�Խ�࣬ԭ�Ӱ뾶Խ��ͬ���ڴ�������ԭ�Ӱ뾶�ڼ�С����ۢܢݢ�ԭ�Ӱ뾶�ɴ�С��˳��ΪNa��Al��S��F���ʴ�Ϊ��Na��Al��S��F��
��3������Ԫ���У�Ԫ�ص�����������Ӧˮ�����и������������ǿ���仯ѧʽΪHClO4��KOH�ļ�����ǿ��Al��OH��3�������ԣ�
�ʴ�Ϊ��HClO4�������KOH��Al��OH��3��
��4��Al�ĵ��Ӳ�Ϊ3������������Ϊ3����ԭ�ӽṹʾ��ͼΪ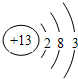 ���ʴ�Ϊ��
���ʴ�Ϊ��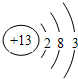 ��
��
��5��ͬ����Ԫ�ش��ϵ��·ǽ������ڼ��������ȵķǽ���ǿ�����ɵ���֮����û���Ӧ��˵������ӦΪCl2+2NaBr=2NaCl+Br2���ʴ�Ϊ���ȣ�Cl2+2NaBr=2NaCl+Br2��
��6����Sn��Ԫ�����ڱ���λ�ÿ�֪��λ�ڵ������ڵ�IVA�壬�ʴ�Ϊ���������ڵ�IVA�壮
��1��Ԫ�������ɵı���ΪԪ��ԭ�Ӻ�������Ų��������Ա仯����Ԫ�����ʵ������Ա仯���ʴ�Ϊ��Ԫ��ԭ�Ӻ�������Ų��������Ա仯��
��2�����Ӳ�Խ�࣬ԭ�Ӱ뾶Խ��ͬ���ڴ�������ԭ�Ӱ뾶�ڼ�С����ۢܢݢ�ԭ�Ӱ뾶�ɴ�С��˳��ΪNa��Al��S��F���ʴ�Ϊ��Na��Al��S��F��
��3������Ԫ���У�Ԫ�ص�����������Ӧˮ�����и������������ǿ���仯ѧʽΪHClO4��KOH�ļ�����ǿ��Al��OH��3�������ԣ�
�ʴ�Ϊ��HClO4�������KOH��Al��OH��3��
��4��Al�ĵ��Ӳ�Ϊ3������������Ϊ3����ԭ�ӽṹʾ��ͼΪ
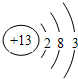 ���ʴ�Ϊ��
���ʴ�Ϊ��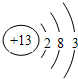 ��
�� ��5��ͬ����Ԫ�ش��ϵ��·ǽ������ڼ��������ȵķǽ���ǿ�����ɵ���֮����û���Ӧ��˵������ӦΪCl2+2NaBr=2NaCl+Br2���ʴ�Ϊ���ȣ�Cl2+2NaBr=2NaCl+Br2��
��6����Sn��Ԫ�����ڱ���λ�ÿ�֪��λ�ڵ������ڵ�IVA�壬�ʴ�Ϊ���������ڵ�IVA�壮
���������⿼��Ԫ�����ڱ���Ԫ�������ɣ���ϤԪ�������ڱ��е�λ���ǽ����Ĺؼ�������Ԫ�ؼ��䵥�ʡ�����������ʼ��ɽ���ѶȲ���

��ϰ��ϵ�д�
 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�
�����Ŀ
 ��
��