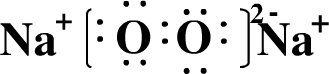��Ŀ����
���ո���ɣ��±��е�ʵ�߱�ʾԪ�����ڱ��IJ��ֱ߽磮��-�ֱ��ʾԪ�����ڱ��ж�Ӧλ�õ�Ԫ�أ�
��1�����ڱ����ô�ʵ�߲�ȫԪ�����ڱ��߽磮
��2������Ԫ���зǽ�������ǿ��Ԫ����
��3��д���ɢܵ��⻯�������⻯�ﷴӦ����ɵĻ�����ĵ���ʽ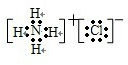
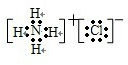 ������Ԫ���е�ijһԪ�ص�����������Ӧ��ˮ������ǿ����ˮ��Һ�ܺ�Ԫ�آĵ��ʷ�����Ӧ���䷴Ӧ����ʽΪ
������Ԫ���е�ijһԪ�ص�����������Ӧ��ˮ������ǿ����ˮ��Һ�ܺ�Ԫ�آĵ��ʷ�����Ӧ���䷴Ӧ����ʽΪ
| �� | |||||||||||||||||
| �� | �� | �� | |||||||||||||||
| �� | �� | �� | |||||||||||||||
| �� | |||||||||||||||||
��2������Ԫ���зǽ�������ǿ��Ԫ����
��
��
������Ԫ�����ƣ��ߵ�ԭ������Ϊ34
34
����3��д���ɢܵ��⻯�������⻯�ﷴӦ����ɵĻ�����ĵ���ʽ
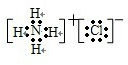
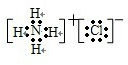
2Al+2H2O+2NaOH=3H2��+2Na[Al��OH��4]
2Al+2H2O+2NaOH=3H2��+2Na[Al��OH��4]
����������1������Ԫ�����ڱ��Ľṹ�Լ�Ԫ�صķֲ����������
��2��ͬһ���ڴ����ң�Ԫ�صĽ������������ǽ�������ǿ��ͬ������ϵ��£�Ԫ�صķǽ�������������������ǿ��
��3�����ݻ�����ijɼ���������д����ʽ�����������Ժ�ǿ�Ӧ��
��2��ͬһ���ڴ����ң�Ԫ�صĽ������������ǽ�������ǿ��ͬ������ϵ��£�Ԫ�صķǽ�������������������ǿ��
��3�����ݻ�����ijɼ���������д����ʽ�����������Ժ�ǿ�Ӧ��
����⣺����Ԫ�����ڱ��Ľṹ��Ԫ�صķֲ�������֪����ΪH����ΪNa����ΪC����ΪN����ΪF������Al����ΪSe����ΪCl��
��1��Ԫ�����ڱ��Ľṹ�Լ�Ԫ�صķֲ��������ʾԪ�����ڱ��IJ��ֱ߽����£�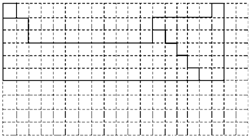 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2��ͬһ���ڴ����ң�Ԫ�صķǽ�������ǿ��ͬ������ϵ��£�Ԫ�صķǽ�������������������ǿ��Ԫ�������ڱ������Ͻǣ�ΪF�������͵������ڣ��ڢ�AԪ�ص�ԭ���������18��SΪ16�ţ�����Se��ԭ������Ϊ34��
�ʴ�Ϊ��F��34��
��3�������Ǽ������壬�Ȼ������������壬���ߵķ�ӦΪ��NH3+HCl=NH4Cl���Ȼ�������ӻ��������ʽΪ��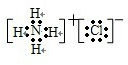 �����������������Ƶķ�ӦΪ��2Al+2H2O+2NaOH=3H2��+2Na[Al��OH��4]��
�����������������Ƶķ�ӦΪ��2Al+2H2O+2NaOH=3H2��+2Na[Al��OH��4]��
�ʴ�Ϊ��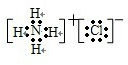 ��2Al+2H2O+2NaOH=3H2��+2Na[Al��OH��4]��
��2Al+2H2O+2NaOH=3H2��+2Na[Al��OH��4]��
��1��Ԫ�����ڱ��Ľṹ�Լ�Ԫ�صķֲ��������ʾԪ�����ڱ��IJ��ֱ߽����£�
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����2��ͬһ���ڴ����ң�Ԫ�صķǽ�������ǿ��ͬ������ϵ��£�Ԫ�صķǽ�������������������ǿ��Ԫ�������ڱ������Ͻǣ�ΪF�������͵������ڣ��ڢ�AԪ�ص�ԭ���������18��SΪ16�ţ�����Se��ԭ������Ϊ34��
�ʴ�Ϊ��F��34��
��3�������Ǽ������壬�Ȼ������������壬���ߵķ�ӦΪ��NH3+HCl=NH4Cl���Ȼ�������ӻ��������ʽΪ��
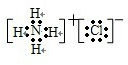 �����������������Ƶķ�ӦΪ��2Al+2H2O+2NaOH=3H2��+2Na[Al��OH��4]��
�����������������Ƶķ�ӦΪ��2Al+2H2O+2NaOH=3H2��+2Na[Al��OH��4]���ʴ�Ϊ��
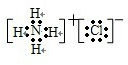 ��2Al+2H2O+2NaOH=3H2��+2Na[Al��OH��4]��
��2Al+2H2O+2NaOH=3H2��+2Na[Al��OH��4]�����������⿼��ѧ��Ԫ�����ڱ���Ԫ�������ɵ��ۺ�֪ʶ��Ҫ��ѧ�����з����ͽ��������������ѶȽϴ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
���ո���ɣ����ֶ�����Ԫ�ص����ʻ�ṹ��Ϣ���±����������Ϣ�ش��������⣮
��1��BԪ�������ڱ��е�λ����______��д��C������ȼ�պ����ĵ���ʽ______��
��2��д��C������ˮ��Ӧ�Ļ�ѧ����ʽ______��A����Ԫ���γɵĻ���������ˮ����Һ��pH______7������ڡ������ڡ���С�ڡ�����
��3��D���ᷴӦ�Ļ�ѧ����ʽΪ______��NaOH��Ӧ�����ӷ���ʽΪ______��
��4��A��B��Ԫ�طǽ����Խ�ǿ���ǣ�дԪ�ط��ţ�______��д��������������Ӧˮ���������ǿ��______�����û�ѧʽ��ʾ��
| Ԫ�� | A | B | C | D |
| ���ʻ� �ṹ ��Ϣ | �����µ���Ϊ��ɫ��ĩ״���壬�������ۻ���������������ȼ�գ���������������ɫ���棬�����д̼�����ζ������ | ���ʳ��¡���ѹ�������壬������ˮ��ԭ�ӵ�M������7������ | ��������������ɫ���塢������ǿ�������ڿ�����ȼ�շ�����ɫ�Ļ��棬���ɵ���ɫ�Ĺ��� | ԭ��������Ӳ���4�����ӣ��䵥�ʼ������ᷴӦ��������Ӧ |
��2��д��C������ˮ��Ӧ�Ļ�ѧ����ʽ______��A����Ԫ���γɵĻ���������ˮ����Һ��pH______7������ڡ������ڡ���С�ڡ�����
��3��D���ᷴӦ�Ļ�ѧ����ʽΪ______��NaOH��Ӧ�����ӷ���ʽΪ______��
��4��A��B��Ԫ�طǽ����Խ�ǿ���ǣ�дԪ�ط��ţ�______��д��������������Ӧˮ���������ǿ��______�����û�ѧʽ��ʾ��
���ո���ɣ��±��е�ʵ�߱�ʾԪ�����ڱ��IJ��ֱ߽磮��-�ֱ��ʾԪ�����ڱ��ж�Ӧλ�õ�Ԫ�أ�
��1�����ڱ����ô�ʵ�߲�ȫԪ�����ڱ��߽磮
��2������Ԫ���зǽ�������ǿ��Ԫ����______������Ԫ�����ƣ��ߵ�ԭ������Ϊ______��
��3��д���ɢܵ��⻯�������⻯�ﷴӦ����ɵĻ�����ĵ���ʽ______������Ԫ���е�ijһԪ�ص�����������Ӧ��ˮ������ǿ����ˮ��Һ�ܺ�Ԫ�آĵ��ʷ�����Ӧ���䷴Ӧ����ʽΪ______��
| �� | |||||||||||||||||
| �� | �� | �� | |||||||||||||||
| �� | �� | �� | |||||||||||||||
| �� | |||||||||||||||||
��2������Ԫ���зǽ�������ǿ��Ԫ����______������Ԫ�����ƣ��ߵ�ԭ������Ϊ______��
��3��д���ɢܵ��⻯�������⻯�ﷴӦ����ɵĻ�����ĵ���ʽ______������Ԫ���е�ijһԪ�ص�����������Ӧ��ˮ������ǿ����ˮ��Һ�ܺ�Ԫ�آĵ��ʷ�����Ӧ���䷴Ӧ����ʽΪ______��