��Ŀ����
��������ʮ�����ʣ�
��H2����������CaO����CO2����H2SO4����Ba(OH)2���ߺ��ɫ����������Һ�壻�ఱˮ����ϡ�����Al2(SO4)3
���������ʰ����ʵķ������д����Ŀհ״��������ʱ�ţ���
| ����� | �������� | ������ | ��Һ | ���� | ����� |
| ���ڸ�������� | | | | | |
��2������ʮ������������������֮��ɷ������ӷ�Ӧ��H����OH��= H2O�������ӷ�Ӧ��Ӧ�Ļ�ѧ����ʽΪ ��
��3������ˮ�еĵ��뷽��ʽΪ ��
��4�������Ģ�ͨ�����Һ�з�Ӧ�����ӷ���ʽΪ ��
��5������ᷢ����Ӧ�Ļ�ѧ����ʽΪ��Al + 4HNO3 = Al(NO3)3 + NO�� + 2H2O���÷�Ӧ���������� ���ѧʽ�����������뻹ԭ�������ʵ���֮���� ������5��4g Al������Ӧʱ��ת�Ƶ��ӵ����ʵ���Ϊ ���÷�Ӧ�����ӷ���ʽΪ ��
��1�� �������� ������ ��Һ ���� ����� ���ڸ�������� �� �ۢ� ��� �� �ۢݢޢ�
��2��Ba(OH)2 + 2HNO3 = Ba(NO3)2 + 2H2O
��3��Al2(SO4)3 = 2Al3��+ 3SO42��
��4��Ba2��+2OH��+CO2 = BaCO3��+ H2O
��5��HNO3 1�U1 0��6 mol Al + 4H��+NO3�� =Al3��+NO��+ 2 H2O
���������������1��������ֻ�н�����Ԫ����ɵĽ������ʣ���Na2O�͢�CO2�к�������Ԫ������һ��Ԫ������Ԫ�أ�����������ఱˮ�к���NH3��H2O��NH3��H2O���������ǻ�����ϡ�������H+��NO3-��H2O�����������ڻ�����������ֱ��С��1nm,����������Һ���ߺ��ɫ����������Һ������Fe(OH)3��ֱ����1��100nm֮�䣬���ڽ��塣�ۢݢޢ���ˮ��Һ��������״̬���ܵ��磬�����Ļ��������ڵ���ʣ��ʴ�Ϊ������� �������� ������ ��Һ ���� ����� ���ڸ�������� �� �ۢ� ��� �� �ۢݢޢ�
��2������ʮ������������������֮��ɷ������ӷ�Ӧ��H++OH-�TH2O������������Ϊ�����Ե�ǿ����ǿ�ᷴӦ���ɿ����Ե��εķ�Ӧ������������µķ�Ӧ����ʽΪ��Ba��OH��2+2HNO3�TBa��NO3��2+2H2O��
��3��Al2(SO4)3 = 2Al3��+ 3SO42��
��4�������Ķ�����̼���Ժ�ǿ������������Ӧ����̼�ᱵ��ˮ�������ӷ���ʽΪ��Ba2++2OH-+CO2=BaCO3��+H2O��
��5����֪Al+4HNO3=Al��NO3��3+NO��+2H2O��HNO3��NO����Ԫ�ػ��ϼ۴�+5����Ϊ+2��HNO3����������1molAl��0������Ϊ+3�ۣ�ʧȥ3mol���ӣ�
1molHNO3���ϼ۴�+5����Ϊ+2�õ�3mol���ӣ����Ի�ԭ���������������ʵ�����ȣ�n��Al��=n/M=5��4g/27g/mol=0��2mol������ת�Ƶ��ӵ����ʵ���Ϊ0��2mol��3=0��6mol��
Al��4HNO3��Ӧ�����ӷ���ʽΪAl+4H++NO3-=Al3++NO��+2H2O��
���㣺�������ʵķ��ࡢ���ʵ����ļ����Լ����ʵ����ʷ���֪ʶ��

�����ڸ�ѹ�¸����������Ⱥ�����ȴ���ɵøֻ�ɫ���塪�����ף���ת���������£�����  ���ף����ױȰ����ȶ����ṹ��ʯī���ơ�����������ȷ����
���ף����ױȰ����ȶ����ṹ��ʯī���ơ�����������ȷ����
| A������ת��Ϊ���������ȷ�Ӧ |
| B�����������Ϊͬ���칹�� |
| C������ת��Ϊ������������ԭ��Ӧ |
| D�������ܵ��� |
����������һ����Ҫ�Ļ���ԭ�ϣ������Ʊ�һϵ�����ʡ�����˵���������( )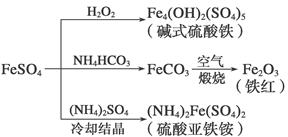
| A����ʽ������ˮ���ܲ���Fe(OH)3���壬��������ˮ�� |
| B��Ϊ��ֹNH4HCO3�ֽ⣬����FeCO3���ڽϵ��¶��½��� |
| C������KSCN��Һ����(NH4)2Fe(SO4)2�Ƿ����� |
| D�������£�(NH4)2Fe(SO4)2��ˮ�е��ܽ�ȱ�FeSO4�Ĵ� |
��1������˵������ȷ����________(����ĸ���)��
| A��60��������ı���HQE�����������ȫ���������������������ڼ��������� |
| B�����ȵĴ�����Һϴ��մ�����۵�����ʱ��������Ҫ�ǻ�ѧ�仯 |
| C��Ӣ�������ѧ�Ҹ�����ڡ�������ά�еĴ���Ӧ���ڹ�ѧͨ�ŷ��桱������ͻ���Գɾͣ��������2009��ŵ��������ѧ����������Ʒ�Ļ���ԭ��ΪSiO2 |
| D��Һ����Һ�ȡ�Һ̬�Ȼ��ⶼ�Ƿǵ���� |
F����ѧ�仯ǰ��ԭ�ӵ����ࡢ��Ŀ���䣬������ĿҲ����
��2�����Ƕ�������ʶ�����м��������ʷ��������һ����dz����ɵͼ���������ʶ���̡�
��1887�갢������˹����������ۡ�
��1923�굤��ѧ�Ҳ���˹�غ�Ӣ����ѧ����������������ۡ������ܹ��ͷ�����(������)���κκ���ԭ�ӵķ��ӻ����Ӷ����������������(������)��ϵķ��ӻ����Ӷ��Ǽ���������ۣ��������ӵ�ˮ��Һ�ȿɿ������ֿɿ��������________(����ĸ���)��
AH2O��BNH4+��COH����DHCO3-��ECH3COO����FCl��
��1923��·��˹(Lewis)����˹��������������ܸ������ӶԶ������γɻ�ѧ���������Ǽ�����ܺ͵��ӶԽ�ϵ����ʶ����ᡣ��
��(���ӶԽ�����)����(���ӶԸ�����)����Ӧ����
H��������������[OH]��������HOH
��ָ������������Ӧ�е����
H3BO3��H2O=H����[B(OH)4]��
�÷�Ӧ�еļ���________(�H3BO3����H2O��)��
CuCl2��4NH3=[Cu(NH3)4]2����2Cl��
�÷�Ӧ�е�����________(�CuCl2����NH3��)��
���з�Ӧ�����ӷ���ʽ�������
| A����ˮ�ӵ��廯����Һ�У�2 Br��+ I2=== 2I�� + Br2 |
| B������ͨ��⻯����Һ�У�2I��+ Cl2=== 2Cl��+ I2 |
| C����������ˮ��Cl2+ H2O === 2H����+ Cl��+ ClO�� |
| D����������Һ�еμӵ⻯����Һ��Ag����+�� I����===�� AgI�� |