��Ŀ����
����Ŀ������Ҫ��ش��������⣺
��1��д������������ˮ��Һ�еĵ��뷽��ʽ��
��Ba(OH)2��__________�� ��NaHSO4__________
��д���٢�ˮ��Һ��Ϻ�ǡ�ó�����ȫ�����ӷ���ʽ______________
��2����ͬ���ʵ�����O2��O3��������__________�����Ӹ�����Ϊ __________��������ԭ�ӵĸ�����Ϊ__________
��3�����и������ʵķ�����ᴿ��Ӧѡ��������������һ�֣�����ѡ����ĸ��
A ��Һ B ���� C ��ȡ D ���� E �����ᾧ F ���·ֽ�
�ٷ���CCl4��H2O__________��
�ڳ�ȥ����ʯ��ˮ��������CaCO3��__________��
�۳�ȥCaO������������CaCO3����__________��
�ܴӵ�ˮ����ȡ��__________��
�ݷ���CCl4���е�Ϊ76.75�棩�ͼױ����е�Ϊ110.6�棩��Һ�����__________��
���𰸡�Ba(OH)2 = Ba2����2OH�� NaHSO4 =Na+ + H+ + SO42�C Ba2+ + SO42- + OH- + H+ = BaSO4�� + H2O 2:3 1:1 2: 3 ��3�� A B F C��CA D
��������
��1����Ba(OH)2��������Ba2+��OH-��
��NaHSO4��������Na+��H+��SO42-��
��Ba(OH)2��NaHSO4��Ӧǡ�ó�����ȫ����Ӧ����ʽΪ��Ba(OH)2 + NaHSO4= BaSO4�� + H2O+ NaOH��
��2������n=![]() �������ʵ���֮�ȣ��������ʵ���֮�ȵ��ڷ�����֮�ȼ��������֮�ȣ���һ��������ԭ�Ӹ����ȣ�
�������ʵ���֮�ȣ��������ʵ���֮�ȵ��ڷ�����֮�ȼ��������֮�ȣ���һ��������ԭ�Ӹ����ȣ�
��3���ٷ��벻���ܵ�Һ��ֱ�ӷ�Һ��
��ʯ��ˮΪ����Һ�壬CaCO3���ܣ����˼��ɷ��룻
��CaCO3���·ֽ⼴�ɵõ�CaO��
�ܵ��ڱ���CCl4�е��ܽ��Զ������ˮ�е��ܽ�ȣ�
�ݷ��뻥�ܵġ��е��в���Ļ��Һͨ��������ķ�����
��1����Ba(OH)2��������Ba2+��OH-�����뷽��ʽΪ��Ba(OH)2= Ba2++OH-��
��NaHSO4��������NaHSO4��������Na+��H+��SO42-�����뷽��ʽΪ��NaHSO4= Na++H++SO42-��
��Ba(OH)2��NaHSO4��Ӧǡ�ó�����ȫ����Ӧ����ʽΪ��Ba(OH)2 + NaHSO4= BaSO4�� + H2O+ NaOH�����ӷ���ʽΪ��Ba2+ + SO42- + OH- + H+ = BaSO4�� + H2O��
��2��![]() =
=![]() =
=![]() =
=![]() ��
��![]() =
=![]() =
=![]() ����ԭ�Ӹ�����Ϊ
����ԭ�Ӹ�����Ϊ![]() =
=![]() ��
��
��3����CCl4��H2O�����ܣ�ֱ�ӷ�Һ���ɷ��룻
��ʯ��ˮΪ����Һ�壬CaCO3���ܣ����˼��ɷ��룻
��CaCO3���·ֽ⼴�ɵõ�CaO����˸��·ֽ⼴�ɳ�ȥ��ʯ���е�CaCO3��
�ܵ��ڱ���CCl4�е��ܽ��Զ������ˮ�е��ܽ�ȣ���˿�������ȡ����Һ�ķ������з��룻
��CCl4�ͼױ����ܣ��ҷе����ϴ����������ķ������롣

 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д�����Ŀ���״�����Ҫ�Ļ���ԭ�ϡ��ڴ����������£����úϳ���(��Ҫ�ɷ�ΪCO��CO2��H2)�ϳɼ״�����Ҫ��ѧ��Ӧ���£�
��CO+2H2CH3OH
��CO2+3H2CH3OH+H2O
��CO2+H2CO+H2O
��ش��������⣺
��1����֪�������ʵı�ȼ�������±���
���� | CO(g) | H2(g) | CH3OH(l) |
ȼ����(kJ��mol1) | 283.0 | 285.8 | 726.51 |
����д25�桢101kPa����ʱCOȼ���ȵ��Ȼ�ѧ����ʽ______________________��
�ڼ���25�桢101kPa����ʱ��Ӧ�����H=_____kJ��mol1 ��
��2���״�ȼ�ϵ�أ�Direct Methanol Fuel Cell���������ӽ���Ĥȼ�ϵ�أ��乤��ԭ����ͼ��ʾ��
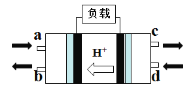
��c��������������________��
�ڸ����ĵ缫��Ӧʽ��____________��
��3����ͼ�Ǽ״�ȼ�ϵ�ع���ʾ��ͼ������A��B��D��Ϊʯī�缫��CΪͭ�缫������һ��ʱ��Ͽ�K����ʱA��B�����ϲ��������������ͬ��
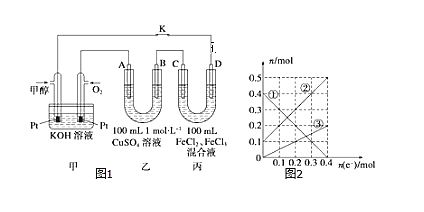
������B���ĵ缫��Ӧʽ_______��
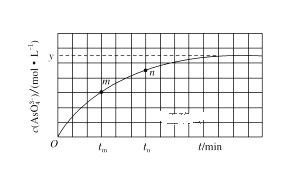
������A�������������ڱ���µ����________��
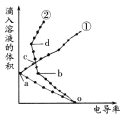
�۱�װ����Һ�н��������ӵ����ʵ�����ת�Ƶ��ӵ����ʵ����仯��ϵ��ͼ������߱�ʾ����____�ı仯����Ӧ������Ҫʹ��װ���н���������ǡ����ȫ��������Ҫ____mL 5mol/LNaOH��Һ��
����Ŀ�����𡢵����ס�ͭ��п�Ļ�������������Ҫ��;���ش��������⣺
��1����̬Bԭ�ӵ���ռ������ܼ��ĵ���������ͼΪ____����̬Cu+�ĺ�������Ų�ʽΪ___��
��2��������(CH3)3N������ˮ��ԭ����______��
��3�������ᣨH3PO3������Ԫ�ص�һ�ֺ����ᣬ��NaOH��Ӧֻ����NaH2PO3��Na2HPO3�����Σ���H3PO3���ӵĽṹʽΪ____��
��4��Zn2+����CN�����������꣨![]() �����γ��ȶ�����
�����γ��ȶ�����
��CN�� �ĽṹʽΪ_____��
��ÿ��������������У���ȡsp2�ӻ���ԭ����__����
��5��±��п���۵������ʾ��
ZnF2 | ZnCl2 | ZnBr2 | ZnI2 | |
�۵�/�� | 872 | 275 | 394 | 446 |
ZnF2���۵�Զ������������±��п����ԭ��Ϊ_____��
��6��������һ����ĥͿ�ϣ��������������ı��汣���㡣�����徧����ͼ1��ʾ��
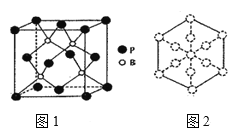
������������Խ��߷����ͶӰ��ͼ2�����ڴ���Ͻ���ʾBԭ�ӵ�ԲȦͿ��____��
����֪��������ܶ�Ϊ�� g/cm3�������ӵ�����ΪNA����B��P����Ϊ___pm���г�����ʽ���ɣ���