��Ŀ����
ij��ѧ����С���ij����������Ʒ��������²������ʵ�飺��ȡһ�����к��ʽ����ҽྻ�������500 mL��ƿ���þ�ȷ��Ϊ0.001 g�ķ�����ƽ(��ͬ)ȷ������������Ϊm1��
������ƿ��ͨ�������ĸ���ĸû��������Ʒ������ƿ����ȷ�������õ�����Ϊm2��
������ƿ�м���ˮ������ƿ��������������Ϊm3��
��ˮ���ܶ�Ϊ��ˮ g��cm-3���������ܶ�Ϊ������ g��cm-3������ʽ��Ϊ29.0����û����Ʒ��ƽ����Է�������Ϊ��29��![]() ��
��
������������⣺
(1)ʵ��Ŀ��_____________________________________________________________��
(2)�������ƿ�п�����������m����=__________������ƿ����Ʒ������m��Ʒ=_________��
(3)�������ۣ�
����һ��ʵ����ɺ��е�ͬѧ�����ʵ�鲻���ڱ�״���½��еģ�Ӧ�ü�¼ʵ����¶�(t ��)��ѹǿ(p kpa)�������й����ݽ��л��㣬����Ϊ�Ƿ��б�Ҫ����˵�����ɡ�
��_________________________________________________________________��
���������ʦ�Ը�С���ʵ����Ƶ���ʱָ����Ϊ��ʹʵ�����ݺ�����Ч����ÿ�β���ʱ����ƿ�е�����(��ˮ)�����Ӧ����ȡ��Դˣ�����Ϊ��ȡ�IJ���Ϊ___________________
_____________________________________________________________________��
(1)�ⶨij����������Ʒ��ƽ����Է�������
![]()
(3)û��Ҫ����Ϊ�òⶨ����������Ʒ����Է������������õ�ԭ��������ͬ�¡�ͬѹ��ͬ���ʱ��������Ʒ�������ȵ�����Է�������֮�ȣ�ֻҪ����ͬһʵ�������½��еľͿ��� �ڵ�1������ʱ����ƿ������������ƿ����������Ǻţ��Ժ�ÿ�γ�������������ƿ�ڵ�λ���Դ�Ϊ��
��������ʵ�鲽��٢ڢۿɵ���Ʒ�������ɢܿɵ���Ʒ��ƽ����Է�����������������Ҳֻ�������Ʒ��ƽ����Է��������������ʵ��Ŀ�ľ��Dzⶨ���������Ʒ��ƽ����Է�������

 ������ҵ����ν�����������ϵ�д�
������ҵ����ν�����������ϵ�д�I��ij��ѧ����С�����Ⱦ�����IJ��ַǽ������������̽�����������ĿҪ��ش��������⡣
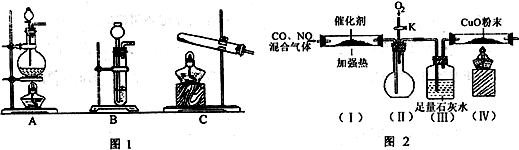
(1)д����������ȡNO�����ӷ���ʽ ��
(2)�����ϵ�֪��HCOOH CO+H2O��ʵ��������ͼl��ʾ��װ�ã���ȡCO�����ѡ�õ�װ��Ϊ
CO+H2O��ʵ��������ͼl��ʾ��װ�ã���ȡCO�����ѡ�õ�װ��Ϊ
(�����)��ʵ�������ø�װ�û�����ȡ�ij��������� ��дһ������ķ���ʽ����
(3)�����ϵ�֪�����ô�����ʹ����β���е�һ����̼�͵�������ַ�����Ӧת��Ϊ������̼�͵�������С����ʵ����ģ������β���������������ͼ2��ʾװ��(���ּгֺ�װ������ȥ)��


��ʵ��ǰ���ر�����K����ͨ�����ž�װ���еĿ�������Ŀ���� ��
��װ��(III)����Ҫ������ ��
�۸���װ�����в�����֮������Ӧ��װ��(��)�� װ�á�
II���ÿ���С����ƵĴ�ZnSO4��FeCl3�Ļ����Һ����ȡZnSO4��7H2O�������£�
a���ڻ��Һ�м���6 mol/L NaOH��Һ����pH=8Ϊֹ��
b�����˺�õ�������������ˮ���ϴ�ӳ�����
c����ϴ�Ӻ�ij����м���2 mol/L�����ᣬ������Һ��pH��4��6��������У����ȹ��ˣ���Һ��ΪZnSO4��Һ��
d����Һ�м���2 mol/L�����ᣬʹ��pH=2��
��֪�����������������������ʽ��ʼ��������ȫ����ʱ��Һ��pH���±����ش��������⣺
|
������ |
Fe(OH)3 |
Zn(OH)2 |
|
pH |
1.5��3.2 |
6.4��8.0 |
(1)����b����μ������Ѿ�ϴ�Ӹɾ� ��
(2)����d�м������ᣬʹ��pH=2��Ŀ���� ��Ҫ�Ƶ�ZnSO4��7H2O�IJ���d��ȱ�ٵIJ����� �����õ���Ҫ������������ ��
��1��д����������ȡһ�����������ӷ���ʽ______��
��2�������ϵ�֪��HCOOH
 CO��+H2O��ʵ��������ͼ1��ʾ��װ�ã���ȡCO�����ѡ�õ�װ��Ϊ______������ţ���ʵ�������ø�װ�û�����ȡ�ij���������______����дһ������ķ���ʽ����
CO��+H2O��ʵ��������ͼ1��ʾ��װ�ã���ȡCO�����ѡ�õ�װ��Ϊ______������ţ���ʵ�������ø�װ�û�����ȡ�ij���������______����дһ������ķ���ʽ������3�������ϵ�֪�����ô�����ʹ����β���е�һ����̼�͵�������ַ�����Ӧת��Ϊ������̼�͵�������С����ʵ����ģ������β���������������ͼ2��ʾװ�ã����ּгֺ�װ������ȥ����

��ʵ��ǰ�ر�����K����ͨ�����ž�װ���еĿ�������Ŀ����______��
��װ�ã�����Ҫ������______��
�۸���װ�����в�����֮������Ӧ��װ�ã�������______װ�ã�
II���ÿ���С����ƵĴ�ZnSO4��FeCl3�Ļ����Һ����ȡZnSO4?7H2O�������£�
a���ڻ��Һ�м���6mol?L-1 NaOH��Һ����pH=8Ϊֹ��
b�����˺�õ�������������ˮ���ϴ�ӳ�����
c����ϴ�Ӻ�ij����м���2mol?L-1�����ᣬ������Һ��pH��4��6��������У����ȹ��ˣ���Һ��ΪZnSO4��Һ��
d����Һ�м���2mol?L-1�����ᣬʹ��pH=2��
��֪�����������������������ʽ��ʼ��������ȫ����ʱ��Һ��pH���±����ش��������⣺
| ������ | Fe��OH��3 | Zn��OH��2 |
| pH | 1.5��3.2 | 6.4��8.0 |
��2������d�м������ᣬʹ��pH=2��Ŀ����______�� Ҫ�Ƶ�ZnSO4?7H2O�IJ���d��ȱ�ٵIJ�����______�����õ���Ҫ������������______��