��Ŀ����
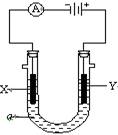
��ͼװ�ã�X��Y��ֱ����Դ���������ֱ�������и���ʵ�飬���±������и����Ӧ��ϵ����ȷ��һ���ǣ�
| ѡ�� | ��ԴX�� | ʵ��ǰU�� ����Һ�� | ͨ��������� |
| A | ���� | Na2SO4��Һ | U�ι����˵����̪��a���гʺ�ɫ |
| B | ���� | AgNO3��Һ | b���е缫��Ӧʽ�� 4OH�� -4e-=2H2O+O2�� |
| C | ���� | KCl��CuCl2�����Һ | ��ͬ�����£�a��b�����в��������������������� |
| D | ���� | Fe(OH)3����͵���Һ | b����Һ����ɫ���� |
C
�������⿼��绯ѧ֪ʶ�������������Һ��ʵ���ǵ��ˮ��H+���������ŵ磬OH-���������ŵ磬�ٽ���ˮ�ĵ��룬�����������д�����OH-,�����̪��b���гʺ�ɫ��A��������������Ag+�������ŵ磬�缫��ӦʽΪ�� 4Ag+ +4e- ==4Ag��OH-��������Ӧ��4OH- +4e- ==2H2O+O2����B����������������������ɣ���A����Һ����ɫ���D�����

��ϰ��ϵ�д�
�����Ŀ

 __________��
__________��
 ����Դ��������ش��������⣺
����Դ��������ش��������⣺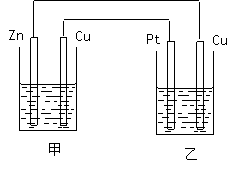 ����������װ���У���װ���� ��ԭ��ػ���أ�������ͭ�缫�ϵĵ缫��ӦΪ
����������װ���У���װ���� ��ԭ��ػ���أ�������ͭ�缫�ϵĵ缫��ӦΪ