��Ŀ����
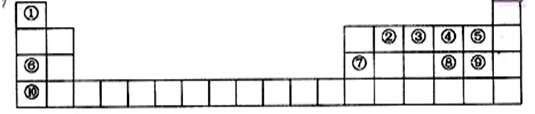
��11�֣��±���Ԫ�����ڱ���һ���֡��������е���ĸ�ֱ����һ�ֻ�ѧԪ�ء�
��1����������õĽ���������õķǽ����γɵĻ�ѧʽ��_______������ �������Ӽ������ۼ���
��2���ߺ�Ԫ�ص�ԭ�ӽṹʾ��ͼΪ___________���䵥�ʼ������ᷴӦ��������Ӧ��д����Ӧ�Ļ�ѧ����ʽ��_________________________________________��
��3���ڡ��ۡ������ۺ������������ǿ������˳���� ��д��ѧʽ����
�ܡ��ݡ����⻯���ȶ�����ǿ������˳���� ��д��ѧʽ����
��4���١��ܡ�������Ԫ���γɵĻ������л�ѧ�������ͣ� ���仯��������Ϊ
������ۻ�������ӻ����
��5���ں͢��γɵĻ�������ܺ͢��γɵĻ�����֮�䷢��������ԭ��Ӧ��д���÷�Ӧ��
��ѧ����ʽ�� ��

��1����������õĽ���������õķǽ����γɵĻ�ѧʽ��_______������ �������Ӽ������ۼ���
��2���ߺ�Ԫ�ص�ԭ�ӽṹʾ��ͼΪ___________���䵥�ʼ������ᷴӦ��������Ӧ��д����Ӧ�Ļ�ѧ����ʽ��_________________________________________��
��3���ڡ��ۡ������ۺ������������ǿ������˳���� ��д��ѧʽ����
�ܡ��ݡ����⻯���ȶ�����ǿ������˳���� ��д��ѧʽ����
��4���١��ܡ�������Ԫ���γɵĻ������л�ѧ�������ͣ� ���仯��������Ϊ
������ۻ�������ӻ����
��5���ں͢��γɵĻ�������ܺ͢��γɵĻ�����֮�䷢��������ԭ��Ӧ��д���÷�Ӧ��
��ѧ����ʽ�� ��
(1)KF ���� ��2��Al�� 2Al+2NaOH+6H2O="2Na" [ Al(OH)4]+3H2��(3)HClO4 HNO3 H2CO3 HF H2O H2S
(4)���Ӽ����ۼ� ���ӻ����� ��5��2Na2O2+2CO2=2Na2CO3+O2
(4)���Ӽ����ۼ� ���ӻ����� ��5��2Na2O2+2CO2=2Na2CO3+O2

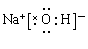
�������и�Ԫ�ص�λ�ã�ȷ����Ԫ�أ�
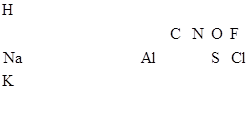
��1������÷ǽ�����F������ý�����K���γ����ӻ�����KF��
��2��
2Al+2NaOH+6H2O="2Na" [ Al(OH)4]+3H2��
��3���ǽ�����Խǿ������Ӧ����ۺ���������Խǿ���ǽ����ԣ�Cl>N>C�����ԣ�HClO4 > HNO3 > H2CO3 ���ǽ�����Խǿ����̬�軯��Խ�ȶ����ǽ����ԣ�F>O>S����̬�⻯���ȶ��ԣ�HF > H2O > H2S��
��4��
�Ⱥ����Ӽ��ֺ����ۼ����������ӻ����
��5��2Na2O2+2CO2=2Na2CO3+O2

��ϰ��ϵ�д�
�����Ŀ