��Ŀ����
����Ŀ���״����е�65������һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ����
��1�������£�1g�״���ȫȼ������Һ̬ˮʱ�ų�22.7kJ��������д����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽ ________________________________________________��
��2�����з�ӦCO(g)��2H2(g) =CH3OH(g)�����������仯��ͼ��ʾ��д���÷�Ӧ���Ȼ�ѧ����ʽ_______________________________��
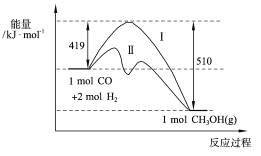
�÷�Ӧ�ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ�� K��250����____K��350��������������������С������,
�����º����£���3molCO��6molH2�����ܱ������н��и÷�Ӧ����Ӧ�ﵽƽ�⣬���������ѹǿΪ��ʼʱ��0.6����CO��ת����Ϊ_______��
��3���Լ״�������Ϊԭ�ϣ�KOH��Һ��Ϊ����ʹ���ȼ�ϵ���ܷ�ӦΪ��2CH3OH+3O2+4OH- =2CO32-+6H2O�����ĵ缫��ӦʽΪ��________________________��
��4������Ը�ȼ�ϵ��Ϊ��Դ��ʯī��������ⱥ��ʳ��ˮ����õ������������ĵ缫��ӦʽΪ��_____________��������һ��ʱ���NaC1��Һ�����Ϊ1L����Һ�е�OH-���ʵ���Ũ��Ϊ0.01 molL-1��25���²ⶨ�������������������������Ϊ_________mL������£���
���𰸡� CH3OH(l)+3/2O2(g)=CO2(g)+2H2O(l) ��H=��726.4kJ/mol CO(g)��2H2(g)===CH3OH(g)����H����91 kJ��mol��1 ���� 60% CH3OH+8OH--6e-=CO32-+6H2O 2Cl--2e-=Cl2�� 56
��������(1). �״��ķе���65���������¼״�ΪҺ�壬1g�״���ȫȼ������Һ̬ˮʱ�ų�22.7kJ����������1mol�״���ȫȼ������Һ̬ˮʱ�ų�������Ϊ��22.7kJ��32=726.4kJ�����Ա�ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪCH3OH(l)+3/2O2(g)=CO2(g)+2H2O(l) ��H=��726.4kJ/mol���ʴ�Ϊ��CH3OH(l)+3/2O2(g)=CO2(g)+2H2O(l) ��H=��726.4kJ/mol��
(2). ��ͼ��֪����ӦCO(g)��2H2(g) =CH3OH(g)����H=��(510��419)kJ/mol=��91kJ��mol��1����÷�Ӧ���Ȼ�ѧ����ʽΪ��CO(g)��2H2(g)===CH3OH(g)����H����91kJ��mol��1����CO(g)��2H2(g) =CH3OH(g)Ϊ���ȷ�Ӧ�������¶ȣ�ƽ�������ƶ�����ѧƽ�ⳣ����С����K��250��������K��350�����������º����£���3molCO��6molH2�����ܱ������н��и÷�Ӧ����Ӧ�ﵽƽ��ʱ�����������ѹǿΪ��ʼʱ��0.6�����ں��º��ݵ������£������������ѹǿ�ȵ����������ʵ���֮�ȣ�����ƽ������ʽ���У�
CO(g)��2H2(g) =CH3OH(g)
��ʼ��(mol) 3 6 0
ת����(mol) x 2x x
ƽ����(mol) 3��x 6��2x x
��![]() =0.6�����x=1.8mol������CO��ת����Ϊ
=0.6�����x=1.8mol������CO��ת����Ϊ![]() ��100%=60%���ʴ�Ϊ��CO(g)��2H2(g)===CH3OH(g)����H����91kJ��mol��1��������60%��
��100%=60%���ʴ�Ϊ��CO(g)��2H2(g)===CH3OH(g)����H����91kJ��mol��1��������60%��
(3). �Լ״�������Ϊԭ�ϣ�KOH��Һ��Ϊ����ʹ���ȼ�ϵ�����ܷ�ӦΪ��2CH3OH+3O2+4OH- =2CO32-+6H2O���ڼ��������£�ȼ�ϵ�ص�������ӦʽΪ3O2��12e����6H2O=12OH���������ܷ�Ӧʽ��ȥ������Ӧʽ�ø�����ӦʽΪ��CH3OH+8OH�D�D6e��=CO32��+6H2O���ʴ�Ϊ��CH3OH+8OH�D�D6e��=CO32��+6H2O��
(4). �Ը�ȼ�ϵ��Ϊ��Դ��ʯī��������ⱥ��ʳ��ˮ����������������ʧ�������������������ĵ缫��ӦʽΪ��2Cl��-2e��=Cl2�����õ��ص�������ӦʽΪ��2H2O��2e��=H2����2OH�������һ��ʱ���NaC1��Һ�����Ϊ1L����Һ�е�OH�����ʵ���Ũ��Ϊ0.01 molL-1��25���²ⶨ��������Һ��OH�����ʵ���Ϊ1L��0.01 molL-1=0.01mol����������Ӧʽ2H2O��2e��=H2����2OH����֪����·��ת�Ƶ��ӵ����ʵ���Ϊ0.01mol�����ݵ�ص�������Ӧʽ3O2��12e����6H2O=12OH�������������ı�״�������������Ϊ0.01mol��4��22.4mol/L=0.056L=56mL���ʴ�Ϊ��2Cl��-2e��=Cl2����56��

