��Ŀ����
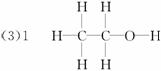
Ϊ�˲ⶨ�Ҵ��������м����ɱ�ȡ����Hԭ�ӣ���ʹ�Ҵ������Na��Ӧ��ʹ���ɵ�H2�ų�ˮ������ų�ˮ������ɼ������״����H2������������������Ҵ������ʵ�������ȷ���Ҵ��������ܱ�ȡ����Hԭ�Ӹ�����
ͼ6-4
(1)����ͼ6-4�����������͵�����װʵ��װ�ã���������������������ʱ�������͵������ӵ�˳����________��________��________��________��________��________��(����)
(2)�������Ӻú����ʵ��ʱ�����в�������������C��װ��
(3)�����Ƶõ�H2�к���ʲô��������?Ӧ��������ȥ?
(4)��ʵ��ʱ����2.9 mL��ˮ�Ҵ�(�ܶ�Ϊ
�������Ҵ���Na�ķ�ӦӦ��װ��C�н��У�CӦ����˫������һ�ײ�F�μ��Ҵ�����һ�ײ嵼����A����H2��H2Ӧ����ʢ��ˮ��Eװ�õ�Һ���ϣ���E�е�ˮ������Bѹ��D�У�����������ʱ��D��ˮ�����Լ����Na���Ҵ���Ӧ���ų�H2�������
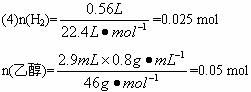
��n(�Ҵ�):n(H2)=0.05 mol:0.025 mol=2:1
��ÿ���Ҵ�������ֻ��1����ԭ�ӿɱ���ȡ����
�𰸣�(1)F C A E B D (2)�ڢܢ٢ۢޢ�
(3)�����Ҵ�����������ˮϴ�ӳ�ȥ�� (4)1