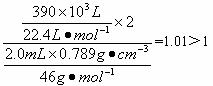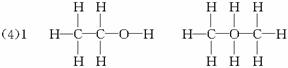��Ŀ����
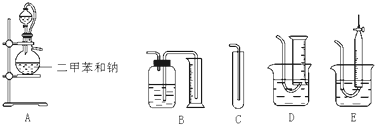
Ϊ�˲ⶨ�Ҵ��Ľṹʽ���������������ˮ�ƾ����Ʒ�Ӧ��ʵ��װ�úͲⶨ���������װ�ý���ʵ�飮�ɹ�ѡ�õ�ʵ��������ͼ��ʾ����ش��������⣺
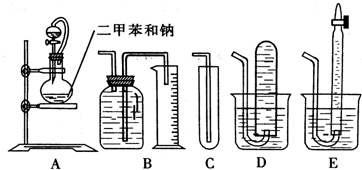
��1�����������������ȷװ����
��2��װ����A���ֵķ�Һ©����������ƿ֮�����ӵĵ��������������
a����ֹ��ˮ�ƾ��ӷ� b����֤ʵ��װ�ò�©�� c��ʹ��ˮ�ƾ�������
��3��ʵ��ǰԤ�Ƚ�С�����ڶ��ױ����ۻ������ɸ�С���飬��ȴ������ƿ�У���Ŀ����
��4����֪��ˮ�ƾ����ܶ�Ϊd g?cm-1����ȡV mL�ƾ�����Ӧ��ȫ���ƹ��������ռ���a mL��������״�������壮��һ���Ҵ��������ܱ���ȡ��������ԭ����Ϊ
���ɴ˼���ȷ���Ҵ��Ľṹʽ��
��5��ʵ�����ⶨ�Ľ��ƫ�ߣ����������ԭ����
a����Ӧ������̶��� b����ˮ�ƾ��л������״�
c����ˮ�ƾ����Ƶķ�Ӧ������ȫ d����ˮ������װ�õ�������ˮ������

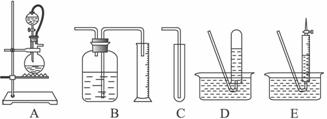
��1�����������������ȷװ����
B
B
������д��ţ���2��װ����A���ֵķ�Һ©����������ƿ֮�����ӵĵ��������������
c
c
��a����ֹ��ˮ�ƾ��ӷ� b����֤ʵ��װ�ò�©�� c��ʹ��ˮ�ƾ�������
��3��ʵ��ǰԤ�Ƚ�С�����ڶ��ױ����ۻ������ɸ�С���飬��ȴ������ƿ�У���Ŀ����
������ˮ�Ҵ����ƵĽӴ������ʹ֮��ַ�Ӧ
������ˮ�Ҵ����ƵĽӴ������ʹ֮��ַ�Ӧ
����4����֪��ˮ�ƾ����ܶ�Ϊd g?cm-1����ȡV mL�ƾ�����Ӧ��ȫ���ƹ��������ռ���a mL��������״�������壮��һ���Ҵ��������ܱ���ȡ��������ԭ����Ϊ
| ||
|
| ||
|
��5��ʵ�����ⶨ�Ľ��ƫ�ߣ����������ԭ����
AB
AB
������д��ţ�a����Ӧ������̶��� b����ˮ�ƾ��л������״�
c����ˮ�ƾ����Ƶķ�Ӧ������ȫ d����ˮ������װ�õ�������ˮ������
��������1�������ܶȱȿ���С�������ſ������ռ���ֻ����ˮ���ռ���D�Թ��̶ȣ�Һ��������������E�еζ����¶�Һ��������������
��2����Һ©����������ƿ֮�����ӵ�����ʹ��ƿ���Һ©����ѹǿ����һ�£�����Һ����£�
��3����������������Լӿ췴Ӧ���ʣ�
��4������2H��H2�����㣻
��5���ⶨ���ƫ�ߵ�ԭ����������ʽ֪���ⶨ�����ƫ�����ʵ���ƫС���������½��л���ˮ�ƾ��л������״���
��2����Һ©����������ƿ֮�����ӵ�����ʹ��ƿ���Һ©����ѹǿ����һ�£�����Һ����£�
��3����������������Լӿ췴Ӧ���ʣ�
��4������2H��H2�����㣻
��5���ⶨ���ƫ�ߵ�ԭ����������ʽ֪���ⶨ�����ƫ�����ʵ���ƫС���������½��л���ˮ�ƾ��л������״���
����⣺��1�������ܶȱȿ���С�������ſ������ռ���ֻ����ˮ���ռ���D�Թ��̶ȣ�Һ��������������E�еζ����¶�Һ������������������ֻ��ѡB����ѡ��B��
��2��A�����з�Һ©����������ƿ֮�����ӵ�����ʹ��ƿ���Һ©����ѹǿ����һ�£���Һ©����Һ����ʹ�Ҵ����ڵ��£���ѡ��C��
��3�������۳�С�飬��Ϊ�������Ҵ����ƵĽӴ��������߷�Ӧ���ʣ�ʹ�Ҵ����Ƴ�ַ�Ӧ���ʴ�Ϊ��������ˮ�Ҵ����ƵĽӴ������ʹ֮��ַ�Ӧ��
��4����ˮ�ƾ������ʵ���Ϊ��
����������ʵ���Ϊ
����2H��H2֪�ܱ���ȡ��������ԭ��Ϊ
��2������һ���Ҵ��������ܱ���ȡ��������ԭ����Ϊ
���ʴ�Ϊ��
��
��5���ⶨ���ƫ�ߵ�ԭ����������ʽ֪���ⶨ�����ƫ�����ʵ���ƫС���������½��л���ˮ�ƾ��л������״�����ѡ��AB��
��2��A�����з�Һ©����������ƿ֮�����ӵ�����ʹ��ƿ���Һ©����ѹǿ����һ�£���Һ©����Һ����ʹ�Ҵ����ڵ��£���ѡ��C��
��3�������۳�С�飬��Ϊ�������Ҵ����ƵĽӴ��������߷�Ӧ���ʣ�ʹ�Ҵ����Ƴ�ַ�Ӧ���ʴ�Ϊ��������ˮ�Ҵ����ƵĽӴ������ʹ֮��ַ�Ӧ��
��4����ˮ�ƾ������ʵ���Ϊ��
| dg?cm-1��VmL |
| 46g/mol |
| a ��10-3L |
| 22.4L/mol |
| a ��10-3L |
| 22.4L/mol |
| ||
|
| ||
|
��5���ⶨ���ƫ�ߵ�ԭ����������ʽ֪���ⶨ�����ƫ�����ʵ���ƫС���������½��л���ˮ�ƾ��л������״�����ѡ��AB��
�������������е��Ѷȵ����⣬�����ڵ���ѧ����ѧϰ��Ȥ������ѧ��ѧϰ��ѧ�Ļ����ԣ�������ж��Է�����Ҳ�ж������㣬����������ѧ���淶�Ͻ���ʵ���������������ѧ����ѧ����������ǿѧ����ѧϰ�����ĺ�ѧϰЧ�ʣ�

��ϰ��ϵ�д�
 ����������������ϵ�д�
����������������ϵ�д�
�����Ŀ