��Ŀ����
����Ŀ����ҵ�ϵ�ⷨ�����������Է�ˮ���õ�����Ni��ԭ����ͼ��ʾ������˵������ȷ��
�ǣ� ��
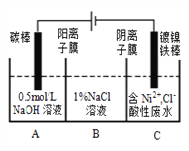
��֪���ŵ�˳��Ni2+(��Ũ��)>H+> Ni2+(��Ũ��)
A. ̼���Ϸ����ĵ缫��Ӧ��4OH--4e-�TO2��+2H2O
B. Ϊ�����Ni�IJ��ʣ�����������Ҫ���Ʒ�ˮpH
C. �������У�B��NaCl��Һ�����ʵ���Ũ�Ƚ���������
D. ����ͼ��������Ĥȥ������A��B���Һϲ������ⷴӦ�ܷ���ʽ���ᷢ���ı�
���𰸡�D
��������A����ͼ֪��̼�����Դ���������ǵ��ص��������缫��Ӧ4OH--4e-=2H2O+O2������A��ȷ��B����Ni2+����������Һ������ˮ�⣻�����ԣ�Ni2+(��Ũ��)��H+��Ni2+(��Ũ��)��Ϊ�����Ni�IJ��ʣ�����������Ҫ���Ʒ�ˮpH����B��ȷ��C�������������Դ���������ǵ��ص��������缫��ӦNi2++2e-=Ni����������Ϊƽ��A��C�еĵ�ɣ�A�е�Na+��C�е�Cl-�ֱ�ͨ��������Ĥ��������Ĥ����B�У���ʹB��NaCl��Һ�����ʵ���Ũ�Ȳ�������C��ȷ��D������ͼ��������Ĥȥ�������ڷŵ�˳��Cl-��OH-����Cl-���������ŵ磺2Cl---2e-=Cl2������ⷴӦ�ܷ���ʽ�ᷢ���ı䣬��D����ѡD��

����Ŀ���±���Ԫ�����ڱ���һ���֣�����ػ�ѧ����ش��������⣺
���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
2 | A | B | C | D | ||||
3 | E | F | G | H | I | J | K | L |
��1��д��H��I����Ԫ�ص�����_____________��_____________��
��2����ЩԪ�ص���̬�⻯�������ȶ�����________________��Ԫ��A�����������ķ���ʽΪ____________������______________����������ӻۣ���
�����ʽΪ_______________����ṹʽΪ_______________��
��3����E��K��Ԫ���У�______ԭ�Ӱ뾶��С��Ԫ��E��J�γɵĻ���������_______����������ӻۣ�
��4������ʵ��֤��E��F�Ľ����Ե�ǿ��������ʵ�����ݺͽ��ۣ�_____________________________________________________________________________________________��