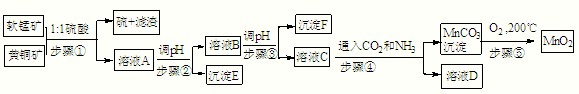
��Ŀ����
��������ͬ�۽������̿���Ҫ�ɷ�MnO2���ͻ�ͭ����Ҫ�ɷ�CuFeS2�����¹��շ�����ȡ�̡�ͭ����Ԫ�ػ�óɹ���������ɫ��ѧ˼�룬��������ʾ�����£���֪��
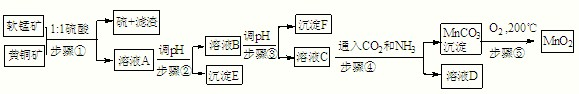
����٣�5MnO2+2CuFeS2+10H2SO4=5MnSO4+Fe2��SO4��3+2CuSO4+4S+10H2O
��1����д��MnO2һ����;��
�����±����ݣ��ش�������⣮
��2��E��
��3����c��MnSO4��=3��10-6 mol?L-1��c��CO32-��=3.3��10-5 mol?L-1����MnSO4��Һ��̼������Һ�������2��1��ϣ�ǡ�ôﵽ�ܽ�ƽ�⣬MnCO3��Ksp=
��4����д������ܷ��������ӷ�Ӧ����ʽ��
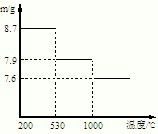
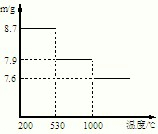
��5������ݣ��ڲ�ͬ�¶��·ֽ�ɵõ��̵IJ�ͬ�����������ͼ���ݼ��㣺530����1000��ʱ���ɵ�һ�ֹ��崿����仯ѧʽΪ��
��6����ȡ0.435g���̿����ձ��У��ٷֱ����ձ��м��������20.00mL 0.1000mol?L-1 Na2C2O4��Һ���������ᣬ��ֽ����ܽ⣬���ˣ�����Һת������ƿ�У���0.1000mol?L-1��KMnO4��Һ�ζ����յ㣬������4.00mL KMnO4����Һ����Ӧʽ���£�MnO2+Na2C2O4+2H2SO4=MnSO4+Na2SO4+2CO2��+2H2O
��д������KMnO4��Һ�ζ�ʣ��Na2C2O4��Һ�����ӷ�Ӧ����ʽ��
�ڼ������̿���MnO2�İٷֺ���

����٣�5MnO2+2CuFeS2+10H2SO4=5MnSO4+Fe2��SO4��3+2CuSO4+4S+10H2O
��1����д��MnO2һ����;��
�������Ʊ�����
�������Ʊ�����
�������±����ݣ��ش�������⣮
| Cu��OH��2 | Fe��OH��3 | Mn��OH��2 | Fe��OH��2 | |
| ��ʼ����pH | 4.2 | 1.5 | 7.3 | 6.4 |
| ��ȫ����pH | 6.7 | 3.2 | 9.8 | 9.0 |
Fe��OH��3
Fe��OH��3
��F��Cu��OH��2
Cu��OH��2
��D����Ҫ������NH4��2SO4
��NH4��2SO4
���ѧʽ������3����c��MnSO4��=3��10-6 mol?L-1��c��CO32-��=3.3��10-5 mol?L-1����MnSO4��Һ��̼������Һ�������2��1��ϣ�ǡ�ôﵽ�ܽ�ƽ�⣬MnCO3��Ksp=
2.2��10-11
2.2��10-11
����4����д������ܷ��������ӷ�Ӧ����ʽ��
Mn2++2NH3+CO2+H2O=MnCO3��+2NH4+
Mn2++2NH3+CO2+H2O=MnCO3��+2NH4+
����5������ݣ��ڲ�ͬ�¶��·ֽ�ɵõ��̵IJ�ͬ�����������ͼ���ݼ��㣺530����1000��ʱ���ɵ�һ�ֹ��崿����仯ѧʽΪ��
Mn2O3
Mn2O3
��
��6����ȡ0.435g���̿����ձ��У��ٷֱ����ձ��м��������20.00mL 0.1000mol?L-1 Na2C2O4��Һ���������ᣬ��ֽ����ܽ⣬���ˣ�����Һת������ƿ�У���0.1000mol?L-1��KMnO4��Һ�ζ����յ㣬������4.00mL KMnO4����Һ����Ӧʽ���£�MnO2+Na2C2O4+2H2SO4=MnSO4+Na2SO4+2CO2��+2H2O
��д������KMnO4��Һ�ζ�ʣ��Na2C2O4��Һ�����ӷ�Ӧ����ʽ��
5C2O42-+2MnO4-+16H+=2Mn2++10CO2��+8H2O
5C2O42-+2MnO4-+16H+=2Mn2++10CO2��+8H2O
���ڼ������̿���MnO2�İٷֺ���
20.0%
20.0%
����������1��������ѧ֪ʶ���ش�������̵����ã�
��2���������ӳ���������ڵ���Һ�����������������ش�
��3������MnCO3��Ksp=[Mn2+]?[CO32-]�����㣻
��4���������������ͨ�������̼�Ͱ��������Ի��̼���̳�����
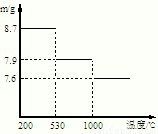
��5������ͼʾ��Ϣ��530���ǻ�õ���8.7g�Ķ������̣���1000���ǵõ�����7.9gMn��������������ش�
��6���ٸ�����ؾ���ǿ�����ԣ����Խ������������
�ڸ�����Ԫ���غ��˼��������������̵İٷֺ�����
��2���������ӳ���������ڵ���Һ�����������������ش�
��3������MnCO3��Ksp=[Mn2+]?[CO32-]�����㣻
��4���������������ͨ�������̼�Ͱ��������Ի��̼���̳�����
��5������ͼʾ��Ϣ��530���ǻ�õ���8.7g�Ķ������̣���1000���ǵõ�����7.9gMn��������������ش�
��6���ٸ�����ؾ���ǿ�����ԣ����Խ������������
�ڸ�����Ԫ���غ��˼��������������̵İٷֺ�����
����⣺��1������ѧ֪ʶ�У��������̵������У���˫��ˮ�ķֽⷴӦ������������ʵ���ҿ����ö������̺�Ũ���ᷴӦ��ȡ�������ʴ�Ϊ���������Ʊ�������
��2�����ݱ������ݣ�����������pH=6.4��ʼ��������pH=9.0ʱ������ȫ��������ͭ��pH=4.2��ʼ��������pH=6.7ʱ������ȫ�����Բ�����Ȼ�õ�������������Ȼ���õ���������ͭ�������Һ������泥�
�ʴ�Ϊ��Fe��OH��3��Cu��OH��2����NH4��2SO4��
��3��MnCO3��Ksp=[Mn2+]?[CO32-]=
��
=2.2��10-11���ʴ�Ϊ��2.2��10-11��
��4���������������ͨ�������̼�Ͱ��������Ի��̼���̳�����ԭ��Ϊ��Mn2++2NH3+CO2+H2O=MnCO3��+2NH4+��
�ʴ�Ϊ��Mn2++2NH3+CO2+H2O=MnCO3��+2NH4+��
��5��530���ǻ�õ���8.7g�Ķ������̣����ʵ�����0.1mol����1000���ǵõ�����7.9gMn���������Է�������=
=79����79����������������Mn2O3���ʴ�Ϊ��Mn2O3��
��6���ٸ�����ؾ���ǿ�����ԣ����Խ������������ʵ���ǣ�C2O42-+2MnO4-+16H+=2Mn2++10CO2��+8H2O��
�ʴ�Ϊ��C2O42-+2MnO4-+16H+=2Mn2++10CO2��+8H2O��
��0.1000mol?L-1��KMnO4��Һ�ζ����յ㣬������4.00mL KMnO4����Һ��������Ƶ�ʣ����=0.1000mol?L-1��0.004L��
=0.001mol�����Է�Ӧ���IJ����Ƶ�����0.001mol�����ݷ�ӦMnO2+Na2C2O4+2H2SO4=MnSO4+Na2SO4+2CO2��+2H2O��
0.001mol Na2C2O4��Һ���ĵ��Ķ������̵����ʵ���Ϊ0.001mol���������̿���MnO2�İٷֺ���=
��100%=20.0%���ʴ�Ϊ��20%��
��2�����ݱ������ݣ�����������pH=6.4��ʼ��������pH=9.0ʱ������ȫ��������ͭ��pH=4.2��ʼ��������pH=6.7ʱ������ȫ�����Բ�����Ȼ�õ�������������Ȼ���õ���������ͭ�������Һ������泥�
�ʴ�Ϊ��Fe��OH��3��Cu��OH��2����NH4��2SO4��
��3��MnCO3��Ksp=[Mn2+]?[CO32-]=
| 3��10-6��2 |
| 3 |
| 3.3��10-5 |
| 3 |
��4���������������ͨ�������̼�Ͱ��������Ի��̼���̳�����ԭ��Ϊ��Mn2++2NH3+CO2+H2O=MnCO3��+2NH4+��
�ʴ�Ϊ��Mn2++2NH3+CO2+H2O=MnCO3��+2NH4+��
��5��530���ǻ�õ���8.7g�Ķ������̣����ʵ�����0.1mol����1000���ǵõ�����7.9gMn���������Է�������=
| 7.9 |
| 0.1 |
��6���ٸ�����ؾ���ǿ�����ԣ����Խ������������ʵ���ǣ�C2O42-+2MnO4-+16H+=2Mn2++10CO2��+8H2O��
�ʴ�Ϊ��C2O42-+2MnO4-+16H+=2Mn2++10CO2��+8H2O��
��0.1000mol?L-1��KMnO4��Һ�ζ����յ㣬������4.00mL KMnO4����Һ��������Ƶ�ʣ����=0.1000mol?L-1��0.004L��
| 5 |
| 2 |
0.001mol Na2C2O4��Һ���ĵ��Ķ������̵����ʵ���Ϊ0.001mol���������̿���MnO2�İٷֺ���=
| 0.001mol��87g/mol |
| 0.435g |
������������һ�����ʵķ�����ᴼ֪ʶ���ۺ���Ŀ��Ҫ��ѧ�����з����ͽ��������������ۺ��Խ�ǿ���ѶȽϴ�

��ϰ��ϵ�д�
 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д� ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д�
�����Ŀ
��������ͬ�۽������̿���Ҫ�ɷ�MnO2���ͻ�ͭ����Ҫ�ɷ�CuFeS2�����¹��շ�����ȡ�̡�ͭ����Ԫ�ػ�óɹ���������ɫ��ѧ˼�룬��������ʾ�����£���֪��
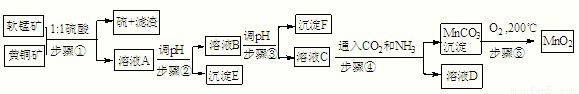
����٣�5MnO2+2CuFeS2+10H2SO4=5MnSO4+Fe2��SO4��3+2CuSO4+4S+10H2O
��1����д��MnO2һ����;��______��
�����±����ݣ��ش�������⣮
��2��E��______��F��______��D����Ҫ����______���ѧʽ����
��3����c��MnSO4��=3×10-6 mol?L-1��c��CO32-��=3.3×10-5 mol?L-1����MnSO4��Һ��̼������Һ�������2��1��ϣ�ǡ�ôﵽ�ܽ�ƽ�⣬MnCO3��Ksp=______��
��4����д������ܷ��������ӷ�Ӧ����ʽ��______��
��5������ݣ��ڲ�ͬ�¶��·ֽ�ɵõ��̵IJ�ͬ�����������ͼ���ݼ��㣺530����1000��ʱ���ɵ�һ�ֹ��崿����仯ѧʽΪ��______��
��6����ȡ0.435g���̿����ձ��У��ٷֱ����ձ��м��������20.00mL 0.1000mol?L-1 Na2C2O4��Һ���������ᣬ��ֽ����ܽ⣬���ˣ�����Һת������ƿ�У���0.1000mol?L-1��KMnO4��Һ�ζ����յ㣬������4.00mL KMnO4����Һ����Ӧʽ���£�MnO2+Na2C2O4+2H2SO4=MnSO4+Na2SO4+2CO2��+2H2O
��д������KMnO4��Һ�ζ�ʣ��Na2C2O4��Һ�����ӷ�Ӧ����ʽ��______��
�ڼ������̿���MnO2�İٷֺ���______��

����٣�5MnO2+2CuFeS2+10H2SO4=5MnSO4+Fe2��SO4��3+2CuSO4+4S+10H2O
��1����д��MnO2һ����;��______��
�����±����ݣ��ش�������⣮
| Cu��OH��2 | Fe��OH��3 | Mn��OH��2 | Fe��OH��2 | |
| ��ʼ����pH | 4.2 | 1.5 | 7.3 | 6.4 |
| ��ȫ����pH | 6.7 | 3.2 | 9.8 | 9.0 |
��3����c��MnSO4��=3×10-6 mol?L-1��c��CO32-��=3.3×10-5 mol?L-1����MnSO4��Һ��̼������Һ�������2��1��ϣ�ǡ�ôﵽ�ܽ�ƽ�⣬MnCO3��Ksp=______��
��4����д������ܷ��������ӷ�Ӧ����ʽ��______��
��5������ݣ��ڲ�ͬ�¶��·ֽ�ɵõ��̵IJ�ͬ�����������ͼ���ݼ��㣺530����1000��ʱ���ɵ�һ�ֹ��崿����仯ѧʽΪ��______��

��6����ȡ0.435g���̿����ձ��У��ٷֱ����ձ��м��������20.00mL 0.1000mol?L-1 Na2C2O4��Һ���������ᣬ��ֽ����ܽ⣬���ˣ�����Һת������ƿ�У���0.1000mol?L-1��KMnO4��Һ�ζ����յ㣬������4.00mL KMnO4����Һ����Ӧʽ���£�MnO2+Na2C2O4+2H2SO4=MnSO4+Na2SO4+2CO2��+2H2O
��д������KMnO4��Һ�ζ�ʣ��Na2C2O4��Һ�����ӷ�Ӧ����ʽ��______��
�ڼ������̿���MnO2�İٷֺ���______��